Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib
- PMID: 26527775
- PMCID: PMC4980567
- DOI: 10.1200/JCO.2015.62.1268
Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib
Abstract
Purpose: Dual human epidermal growth factor receptor 2 (HER2) targeting can increase pathologic complete response rates (pCRs) to neoadjuvant therapy and improve progression-free survival in metastatic disease. CALGB 40601 examined the impact of dual HER2 blockade consisting of trastuzumab and lapatinib added to paclitaxel, considering tumor and microenvironment molecular features.
Patients and methods: Patients with stage II to III HER2-positive breast cancer underwent tumor biopsy followed by random assignment to paclitaxel plus trastuzumab alone (TH) or with the addition of lapatinib (THL) for 16 weeks before surgery. An investigational arm of paclitaxel plus lapatinib (TL) was closed early. The primary end point was pCR in the breast; correlative end points focused on molecular features identified by gene expression-based assays.
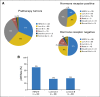
Results: Among 305 randomly assigned patients (THL, n = 118; TH, n = 120; TL, n = 67), the pCR rate was 56% (95% CI, 47% to 65%) with THL and 46% (95% CI, 37% to 55%) with TH (P = .13), with no effect of dual therapy in the hormone receptor-positive subset but a significant increase in pCR with dual therapy in those with hormone receptor-negative disease (P = .01). The tumors were molecularly heterogeneous by gene expression analysis using mRNA sequencing (mRNAseq). pCR rates significantly differed by intrinsic subtype (HER2 enriched, 70%; luminal A, 34%; luminal B, 36%; P < .001). In multivariable analysis treatment arm, intrinsic subtype, HER2 amplicon gene expression, p53 mutation signature, and immune cell signatures were independently associated with pCR. Post-treatment residual disease was largely luminal A (69%).
Conclusion: pCR to dual HER2-targeted therapy was not significantly higher than single HER2 targeting. Tissue analysis demonstrated a high degree of intertumoral heterogeneity with respect to both tumor genomics and tumor microenvironment that significantly affected pCR rates. These factors should be considered when interpreting and designing trials in HER2-positive disease.
Trial registration: ClinicalTrials.gov NCT00770809.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures


Comment in
-
Looking Deep Into the Heterogeneity of Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Can We Understand It Better?J Clin Oncol. 2016 Feb 20;34(6):521-3. doi: 10.1200/JCO.2015.64.7495. Epub 2016 Jan 11. J Clin Oncol. 2016. PMID: 26755511 No abstract available.
References
-
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2–positive metastatic breast cancer: Final results from the EGF104900 study. J Clin Oncol. 2012;30:2585–2592. - PubMed
-
- Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CA33601/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- U10 CA181009/CA/NCI NIH HHS/United States
- UG1 CA233178/CA/NCI NIH HHS/United States
- 1U10CA180801/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- P50 CA058223/CA/NCI NIH HHS/United States
- P50-CA58223/CA/NCI NIH HHS/United States
- U10CA180882/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- 1U10CA180838/CA/NCI NIH HHS/United States
- U10 CA180818/CA/NCI NIH HHS/United States
- 1U10CA180867/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10CA180821/CA/NCI NIH HHS/United States
- 1U10CA180791/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180838/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

