Basal adenosine modulates the functional properties of AMPA receptors in mouse hippocampal neurons through the activation of A1R A2AR and A3R
- PMID: 26528137
- PMCID: PMC4601258
- DOI: 10.3389/fncel.2015.00409
Basal adenosine modulates the functional properties of AMPA receptors in mouse hippocampal neurons through the activation of A1R A2AR and A3R
Abstract
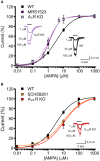
Adenosine is a widespread neuromodulator within the CNS and its extracellular level is increased during hypoxia or intense synaptic activity, modulating pre- and postsynaptic sites. We studied the neuromodulatory action of adenosine on glutamatergic currents in the hippocampus, showing that activation of multiple adenosine receptors (ARs) by basal adenosine impacts postsynaptic site. Specifically, the stimulation of both A1R and A3R reduces AMPA currents, while A2AR has an opposite potentiating effect. The effect of ARs stimulation on glutamatergic currents in hippocampal cultures was investigated using pharmacological and genetic approaches. A3R inhibition by MRS1523 increased GluR1-Ser845 phosphorylation and potentiated AMPA current amplitude, increasing the apparent affinity for the agonist. A similar effect was observed blocking A1R with DPCPX or by genetic deletion of either A3R or A1R. Conversely, impairment of A2AR reduced AMPA currents, and decreased agonist sensitivity. Consistently, in hippocampal slices, ARs activation by AR agonist NECA modulated glutamatergic current amplitude evoked by AMPA application or afferent fiber stimulation. Opposite effects of AR subtypes stimulation are likely associated to changes in GluR1 phosphorylation and represent a novel mechanism of physiological modulation of glutamatergic transmission by adenosine, likely acting in normal conditions in the brain, depending on the level of extracellular adenosine and the distribution of AR subtypes.
Keywords: A3R; AMPA receptors; adenosine receptors; hippocampal neurons; neuronal modulation.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

