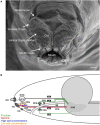
Figure 2
A neuronal map of the larval taste system. The neuronal map defines the single neurons of the larval taste system based on their molecular and functional properties. The peripheral chemosensory system of the larva consists of three major external organs. The DO dome comprises 21 olfactory sensory neurons, which all were shown to express ORs (Fishilevich et al., ; Kreher et al., 2005). There are additional 11 sensory neurons at its base (Sokolowski et al., ; Rohwedder et al., 2012). Of these, two neurons are putatively involved in taste sensing (A1 and A2) due to the expression of GRs (Kwon et al., 2011), and three further neurons mediate thermal stimuli (T1-T3) (Apostolopoulou et al., 2014). We consider the temperature sensitive neurons to be different than the GR-expressing ones, though this was not corroborated by co-localization experiments. The TO can be separated into a dorsolateral group and a distal group consisting of seven and 30 sensory neurons, respectively (Green et al., ; Sokolowski et al., 1984). The dorsolateral group was shown to perceive bitter taste (B1-B2) (Kwon et al., 2011), pheromone (B3) (Wang et al., 2004) and likely mechanosensory information (Green et al., ; Becher et al., 2012). The identity of two sensory neurons remains yet elusive. The distal group was suggested to mainly serve gustatory function. Its sensory neurons sense bitter as well as salt taste (C1-C4) (Niewalda et al., ; Mishra et al., 2013), taste (C5) and CO2 (C6) (Kwon et al., 2007, 2011). The function of further cells is unknown but they are characterized by the expression of IRs (C7 and C8) (Croset et al., 2010). In addition, some PPKs (PPK11, PPK6, PPK23) showed expression in neurons of the TO (Colomb et al., 2007). However, they were not mapped to defined neurons. Co-Expression with GRs is possible, because two PPK receptors, PPK12 and PPK23, were found in neurons that express GR66a, too (Colomb et al., ; Mast et al., 2014). However, the nature of the remaining neurons is unclear. The VO was often excluded from anatomical or functional studies (Sokolowski, ; Kwon et al., 2011). However, from ultrastructural data on house fly and fruit fly larvae gustatory function was derived (Sokolowski et al., ; Python and Stocker, ; Schwarz et al., 2014). Taste sensing is corroborated for at least one neuron due to the expression of a GR, GR2a, (H1) (Colomb et al., 2007). The pharyngeal sensory system of the larva consists of four sensory organs. The DPS provide mainly gustatory function. They house bitter (D1 and D2) (Kwon et al., 2011) and sugar sensing neurons (D3) (Mishra et al., 2013). Additionally, four IRs (IR60e, IR67c, IR60b, and IR94f) are expressed in three neurons (D4–D6) (Stewart et al., 2015). Hence, the identity of 10 additional sensory neurons is unknown. Expression of several other GRs was found in the DPS but not mapped to defined neurons (Kwon et al., 2011). The VPS was assumed to serve gustatory function, too. Bitter (E1 and E2) and sugar sensing neurons (E3) are corroborated. A PPK receptor, PPK6, is expressed in two neurons (E4 and E5) (Chu and Axtell, ; Colomb et al., ; Kwon et al., 2011), and one IR, IR11a, in one neuron (E6) (Croset et al., 2010). Their function is unknown. Because two more neurons were suggested to perceive mechanosensory input (Green et al., ; Sokolowski et al., 1984), the identity of additional nine neurons remains elusive. The DPO consists of only five sensory neurons (Gendre et al., ; Colomb et al., 2007). One neuron (G1) is labeled by the IR20a-Gal4 driver (Stewart et al., 2015). Two further neurons are putatively bitter sensing, due to the expression of GR66a (G2 and G3). Interestingly, one of them co-expressed PPK12 (Colomb et al., 2007). The PPS consists of six sensory neurons that probably serve gustatory function (Python and Stocker, 2002). Two neurons sense bitter (F1 and F2) and another one sweet (F3) (Dethier and Gelperin, ; Chu and Axtell, 1971). Two further neurons can be characterized due to the expression of IR100a (F4 and F6) (Croset et al., 2010). Seven additional GR-Gal4 lines label cells in the PPS (Kwon et al., 2011). In all taste organs, IR76b and IR25a showed expression in a broad number of cells and therefore were assumed to be co-receptors (Croset et al., ; Stewart et al., 2015). The identity of proposed neurons was not in every case definitely corroborated by co-expression studies. Nevertheless, we consider the “bitter” GRs, GR66a and GR33a to be co-expressed in bitter sensing neurons in all organs, though this was only shown for the TO (Kwon et al., ; Apostolopoulou et al., 2014). Further, we assume IR-expressing neurons to be different from GR- and PPK-expressing neurons, because they have not been shown to co-express with these receptor genes, yet. For some receptor genes, e.g., GR66a, the numbers of associated neurons varied in the literature (Colomb et al., ; Kwon et al., 2011). In this case, the lower number was chosen for the presented neuronal map. An exception was GR43a, of which we included the expression data from Mishra et al. (2013).