Lactate does not activate NF-κB in oxidative tumor cells
- PMID: 26528183
- PMCID: PMC4602127
- DOI: 10.3389/fphar.2015.00228
Lactate does not activate NF-κB in oxidative tumor cells
Abstract
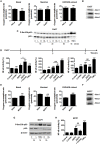
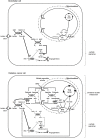
The lactate anion is currently emerging as an oncometabolite. Lactate, produced and exported by glycolytic and glutaminolytic cells in tumors, can be recycled as an oxidative fuel by oxidative tumors cells. Independently of hypoxia, it can also activate transcription factor hypoxia-inducible factor-1 (HIF-1) in tumor and endothelial cells, promoting angiogenesis. These protumoral activities of lactate depend on lactate uptake, a process primarily facilitated by the inward, passive lactate-proton symporter monocarboxylate transporter 1 (MCT1); the conversion of lactate and NAD(+) to pyruvate, NADH and H(+) by lactate dehydrogenase-1 (LDH-1); and a competition between pyruvate and α-ketoglutarate that inhibits prolylhydroxylases (PHDs). Endothelial cells do not primarily use lactate as an oxidative fuel but, rather, as a signaling agent. In addition to HIF-1, lactate can indeed activate transcription factor nuclear factor-κB (NF-κB) in these cells, through a mechanism not only depending on PHD inhibition but also on NADH alimenting NAD(P)H oxidases to generate reactive oxygen species (ROS). While NF-κB activity in endothelial cells promotes angiogenesis, NF-κB activation in tumor cells is known to stimulate tumor progression by conferring resistance to apoptosis, stemness, pro-angiogenic and metastatic capabilities. In this study, we therefore tested whether exogenous lactate could activate NF-κB in oxidative tumor cells equipped for lactate signaling. We report that, precisely because they are oxidative, HeLa and SiHa human tumor cells do not activate NF-κB in response to lactate. Indeed, while lactate-derived pyruvate is well-known to inhibit PHDs in these cells, we found that NADH aliments oxidative phosphorylation (OXPHOS) in mitochondria rather than NAD(P)H oxidases in the cytosol. These data were confirmed using oxidative human Cal27 and MCF7 tumor cells. This new information positions the malate-aspartate shuttle as a key player in the oxidative metabolism of lactate: similar to glycolysis that aliments OXPHOS with pyruvate produced by pyruvate kinase and NADH produced by glyceraldehyde-3-phosphate dehydrogenase (GAPDH), oxidative lactate metabolism aliments OXPHOS in oxidative tumor cells with pyruvate and NADH produced by LDH1.
Keywords: NAD(P)H oxidases (Nox); NADH; cancer metabolism; lactate signaling; malate-aspartate shuttle; mitochondria; nuclear factor-κB; oxidative phosphorylation (OXPHOS).
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

