Real-Time Imaging of Resident T Cells in Human Lung and Ovarian Carcinomas Reveals How Different Tumor Microenvironments Control T Lymphocyte Migration
- PMID: 26528284
- PMCID: PMC4600956
- DOI: 10.3389/fimmu.2015.00500
Real-Time Imaging of Resident T Cells in Human Lung and Ovarian Carcinomas Reveals How Different Tumor Microenvironments Control T Lymphocyte Migration
Abstract
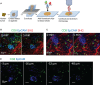
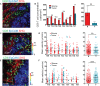
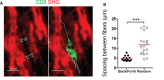
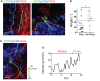
T cells play a key role in the battle against cancer. To perform their antitumor activities, T cells need to adequately respond to tumor antigens by establishing contacts with either malignant cells or antigen-presenting cells. These latter functions rely on a series of migratory steps that go from entry of T cells into the tumor followed by their locomotion in the tumor stroma. Our knowledge of how T cells migrate within tumors mainly comes from experiments performed in mouse models. Whereas such systems have greatly advanced our understanding, they do not always faithfully recapitulate the disease observed in cancer patients. We previously described a technique based on tissue slices that enables to track with real-time imaging microscopy the motile behavior of fluorescent T cells plated onto fresh sections of human lung tumors. We have now refined this approach to monitor the locomotion of resident tumor-infiltrating CD8 T cells labeled with fluorescently coupled antibodies. Using this approach, our findings reveal that CD8 T cells accumulate in the stroma of ovarian and lung carcinomas but move slowly in this compartment. Conversely, even though less populated, tumors islets were found to be zones of faster migration for resident CD8 T cells. We also confirm the key role played by collagen fibers, which, by their orientation, spacing and density, control the distribution and migration of resident CD8 T cells within the tumor stroma. We have subsequently demonstrated that, under some physical tissue constraints, CD8 T cells exhibited a mode of migration characterized by alternate forward and backward movements. In sum, using an ex vivo assay to track CD8 T cells in fresh human tumor tissues, we have identified the extracellular matrix as a major stromal component in influencing T cell migration, thereby impacting the control of tumor growth. This approach will aid in the development and testing of novel immunotherapy strategies to promote T cell migration in tumors.
Keywords: T cells; extracellular matrix; human cancer; imaging; lung cancer; migration; ovarian cancer; stroma.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

