Uncovering the dual role of RHAMM as an HA receptor and a regulator of CD44 expression in RHAMM-expressing mesenchymal progenitor cells
- PMID: 26528478
- PMCID: PMC4606125
- DOI: 10.3389/fcell.2015.00063
Uncovering the dual role of RHAMM as an HA receptor and a regulator of CD44 expression in RHAMM-expressing mesenchymal progenitor cells
Abstract
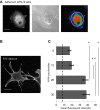
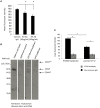
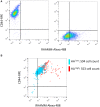
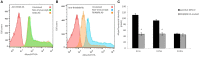
The interaction of hyaluronan (HA) with mesenchymal progenitor cells impacts trafficking and fate after tissue colonization during wound repair and these events contribute to diseases such as cancer. How this interaction occurs is poorly understood. Using 10T½ cells as a mesenchymal progenitor model and fluorescent (F-HA) or gold-labeled HA (G-HA) polymers, we studied the role of two HA receptors, RHAMM and CD44, in HA binding and uptake in non-adherent and adherent mesenchymal progenitor (10T½) cells to mimic aspects of cell trafficking and tissue colonization. We show that fluorescent labeled HA (F-HA) binding/uptake was high in non-adherent cells but dropped over time as cells became increasingly adherent. Non-adherent cells displayed both CD44 and RHAMM but only function-blocking anti-RHAMM and not anti-CD44 antibodies significantly reduced F-HA binding/uptake. Adherent cells, which also expressed CD44 and RHAMM, primarily utilized CD44 to bind to F-HA since anti-CD44 but not anti-RHAMM antibodies blocked F-HA uptake. RHAMM overexpression in adherent 10T½ cells led to increased F-HA uptake but this increased binding remained CD44 dependent. Further studies showed that RHAMM-transfection increased CD44 mRNA and protein expression while blocking RHAMM function reduced expression. Collectively, these results suggest that cellular microenvironments in which these receptors function as HA binding proteins differ significantly, and that RHAMM plays at least two roles in F-HA binding by acting as an HA receptor in non-attached cells and by regulating CD44 expression and display in attached cells. Our findings demonstrate adhesion-dependent mechanisms governing HA binding/ uptake that may impact development of new mesenchymal cell-based therapies.
Keywords: CD44; HMMR; RHAMM; hyaluronan; progenitor.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

