Metformin Is a Substrate and Inhibitor of the Human Thiamine Transporter, THTR-2 (SLC19A3)
- PMID: 26528626
- PMCID: PMC4800991
- DOI: 10.1021/acs.molpharmaceut.5b00501
Metformin Is a Substrate and Inhibitor of the Human Thiamine Transporter, THTR-2 (SLC19A3)
Abstract
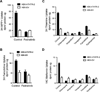
The biguanide metformin is widely used as first-line therapy for the treatment of type 2 diabetes. Predominately a cation at physiological pH's, metformin is transported by membrane transporters, which play major roles in its absorption and disposition. Recently, our laboratory demonstrated that organic cation transporter 1, OCT1, the major hepatic uptake transporter for metformin, was also the primary hepatic uptake transporter for thiamine, vitamin B1. In this study, we tested the reverse, i.e., that metformin is a substrate of thiamine transporters (THTR-1, SLC19A2, and THTR-2, SLC19A3). Our study demonstrated that human THTR-2 (hTHTR-2), SLC19A3, which is highly expressed in the small intestine, but not hTHTR-1, transports metformin (Km = 1.15 ± 0.2 mM) and other cationic compounds (MPP(+) and famotidine). The uptake mechanism for hTHTR-2 was pH and electrochemical gradient sensitive. Furthermore, metformin as well as other drugs including phenformin, chloroquine, verapamil, famotidine, and amprolium inhibited hTHTR-2 mediated uptake of both thiamine and metformin. Species differences in the substrate specificity of THTR-2 between human and mouse orthologues were observed. Taken together, our data suggest that hTHTR-2 may play a role in the intestinal absorption and tissue distribution of metformin and other organic cations and that the transporter may be a target for drug-drug and drug-nutrient interactions.
Keywords: SLC19A3; THTR-2; drug and vitamin interaction; metformin.
Figures






References
-
- Boyle JG, Salt IP, McKay GA. Metformin Action on AMP-Activated Protein Kinase: a Translational Research Approach to Understanding a Potential New Therapeutic Target. Diabet. Med. 2010;27:1097–1106. - PubMed
-
- Tock L, Dâmaso AR, de Piano A, Carnier J, Sanches PL, Lederman HM, Ernandes RMY, de Mello MT, Tufik S. Long-Term Effects of Metformin and Lifestyle Modification on Nonalcoholic Fatty Liver Disease Obese Adolescents. J. Obes. 2010 DOI: 10.1155/2010/831901. - DOI - PMC - PubMed
-
- Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 2011;50:81–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

