A Phase I, Open-Label Trial, Evaluating the Safety and Immunogenicity of Candidate Tuberculosis Vaccines AERAS-402 and MVA85A, Administered by Prime-Boost Regime in BCG-Vaccinated Healthy Adults
- PMID: 26529238
- PMCID: PMC4631471
- DOI: 10.1371/journal.pone.0141687
A Phase I, Open-Label Trial, Evaluating the Safety and Immunogenicity of Candidate Tuberculosis Vaccines AERAS-402 and MVA85A, Administered by Prime-Boost Regime in BCG-Vaccinated Healthy Adults
Abstract
Background: MVA85A and AERAS-402 are two clinically advanced viral vectored TB vaccine candidates expressing Mycobacterium tuberculosis antigens designed to boost BCG-induced immunity. Clinical trials with candidate malaria vaccines have demonstrated that adenoviral vector based priming immunisation, followed by MVA vector boost, induced high levels of immunity. We present the safety and immunogenicity results of the first clinical trial to evaluate this immunisation strategy in TB.
Methods: In this phase 1, open-label trial, 40 healthy previously BCG-vaccinated participants were enrolled into three treatment groups and vaccinated with 1 or 2 doses of AERAS-402 followed by MVA85A; or 3 doses of AERAS-402.
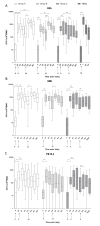
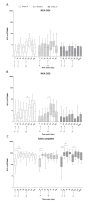
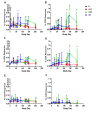
Results: Most related adverse events (AEs) were mild and there were no vaccine related serious AEs. Boosting AERAS-402 with MVA85A significantly increased Ag85A-specific T-cell responses from day of vaccination. Two priming doses of AERAS-402 followed by MVA85A boost, resulted in a significantly higher AUC post-peak Ag85A response compared to three doses of AERAS-402 and historical data with MVA85A vaccination alone. The frequency of CD8+ T-cells producing IFN-γ, TNF-α and IL-2 was highest in the group receiving two priming doses of AERAS-402 followed by MVA85A.
Conclusions: Vaccination with AERAS-402 followed by MVA85A was safe and increased the durability of antigen specific T-cell responses and the frequency and polyfunctionality of CD8+ T-cells, which may be important in protection against TB. Further clinical trials with adenoviral prime-MVA85A boost regimens are merited to optimise vaccination intervals, dose and route of immunisation and to evaluate this strategy in the target population in TB high burden countries.
Trial registration: ClinicalTrials.gov NCT01683773.
Conflict of interest statement
Figures

References
-
- WHO. Global tuberculosis report 2014. Available: http://www.who.int/tb/publications/global_report/en/. Accessed 19 November 2014.
-
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698–702. . - PubMed
-
- Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22(6):1154–8. . - PubMed
-
- WHO. The Stop TB Strategy. Available: http://www.who.int/tb/strategy/stop_tb_strategy/en/. Accessed 19 November 2014.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

