Adipose Mesenchymal Stem Cell Secretome Modulated in Hypoxia for Remodeling of Radiation-Induced Salivary Gland Damage
- PMID: 26529411
- PMCID: PMC4631328
- DOI: 10.1371/journal.pone.0141862
Adipose Mesenchymal Stem Cell Secretome Modulated in Hypoxia for Remodeling of Radiation-Induced Salivary Gland Damage
Abstract
Background and purpose: This study was conducted to determine whether a secretome from mesenchymal stem cells (MSC) modulated by hypoxic conditions to contain therapeutic factors contributes to salivary gland (SG) tissue remodeling and has the potential to improve irradiation (IR)-induced salivary hypofunction in a mouse model.
Materials and methods: Human adipose mesenchymal stem cells (hAdMSC) were isolated, expanded, and exposed to hypoxic conditions (O2 < 5%). The hypoxia-conditioned medium was then filtered to a high molecular weight fraction and prepared as a hAdMSC secretome. The hAdMSC secretome was subsequently infused into the tail vein of C3H mice immediately after local IR once a day for seven consecutive days. The control group received equal volume (500 μL) of vehicle (PBS) only. SG function and structural tissue remodeling by the hAdMSC secretome were investigated. Human parotid epithelial cells (HPEC) were obtained, expanded in vitro, and then irradiated and treated with either the hypoxia-conditioned medium or a normoxic control medium. Cell proliferation and IR-induced cell death were examined to determine the mechanism by which the hAdMSC secretome exerted its effects.
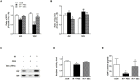
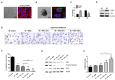
Results: The conditioned hAdMSC secretome contained high levels of GM-CSF, VEGF, IL-6, and IGF-1. Repeated systemic infusion with the hAdMSC secretome resulted in improved salivation capacity and increased levels of salivary proteins, including amylase and EGF, relative to the PBS group. The microscopic structural integrity of SG was maintained and salivary epithelial (AQP-5), endothelial (CD31), myoepithelial (α-SMA) and SG progenitor cells (c-Kit) were successfully protected from radiation damage and remodeled. The hAdMSC secretome strongly induced proliferation of HPEC and led to a significant decrease in cell death in vivo and in vitro. Moreover, the anti-apoptotic effects of the hAdMSC secretome were found to be promoted after hypoxia-preconditioning relative to normoxia-cultured hAdMSC secretome.
Conclusion: These results show that the hAdMSC secretome from hypoxic-conditioned medium may provide radioprotection and tissue remodeling via release of paracrine mediators.
Conflict of interest statement
Figures




References
-
- Dirix P, Nuyts S, Van den Bogaert W (2006) Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer 107: 2525–2534. - PubMed
-
- Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. (2010) A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer 18: 1039–1060. 10.1007/s00520-010-0827-8 - DOI - PubMed
-
- Sasportas LS, Hosford DN, Sodini MA, Waters DJ, Zambricki EA, Barral JK, et al. (2013) Cost-effectiveness landscape analysis of treatments addressing xerostomia in patients receiving head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol 116: e37–51. 10.1016/j.oooo.2013.02.017 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

