Adolescent Intermittent Alcohol Exposure: Deficits in Object Recognition Memory and Forebrain Cholinergic Markers
- PMID: 26529506
- PMCID: PMC4631346
- DOI: 10.1371/journal.pone.0140042
Adolescent Intermittent Alcohol Exposure: Deficits in Object Recognition Memory and Forebrain Cholinergic Markers
Abstract
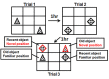
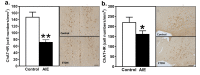
The long-term effects of intermittent ethanol exposure during adolescence (AIE) are of intensive interest and investigation. The effects of AIE on learning and memory and the neural functions that drive them are of particular interest as clinical findings suggest enduring deficits in those cognitive domains in humans after ethanol abuse during adolescence. Although studies of such deficits after AIE hold much promise for identifying mechanisms and therapeutic interventions, the findings are sparse and inconclusive. The present results identify a specific deficit in memory function after AIE and establish a possible neural mechanism of that deficit that may be of translational significance. Male rats (starting at PND-30) received exposure to AIE (5g/kg, i.g.) or vehicle and were allowed to mature into adulthood. At PND-71, one group of animals was assessed using the spatial-temporal object recognition (stOR) test to evaluate memory function. A separate group of animals was used to assess the density of cholinergic neurons in forebrain areas Ch1-4 using immunohistochemistry. AIE exposed animals manifested deficits in the temporal component of the stOR task relative to controls, and a significant decrease in the number of ChAT labeled neurons in forebrain areas Ch1-4. These findings add to the growing literature indicating long-lasting neural and behavioral effects of AIE that persist into adulthood and indicate that memory-related deficits after AIE depend upon the tasks employed, and possibly their degree of complexity. Finally, the parallel finding of diminished cholinergic neuron density suggests a possible mechanism underlying the effects of AIE on memory and hippocampal function as well as possible therapeutic or preventive strategies for AIE.
Conflict of interest statement
Figures

References
-
- Walker DW, Hunter BE (1978) Short-term memory impairment following chronic alcohol consumption in rats. Neuropsychologia 16: 545–553. - PubMed
-
- Riley JN, Walker DW (1978) Morphological alterations in hippocampus after long-term alcohol consumption in mice. Science 201: 646–648. - PubMed
-
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P (1980) Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science 209: 711–713. - PubMed
-
- Swartzwelder HS, Wilson WA, Tayyeb MI (1995) Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res 19: 1480–1485. - PubMed
-
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS (1996) Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res 20: 1346–1351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

