Mitofilin and CHCHD6 physically interact with Sam50 to sustain cristae structure
- PMID: 26530328
- PMCID: PMC4632003
- DOI: 10.1038/srep16064
Mitofilin and CHCHD6 physically interact with Sam50 to sustain cristae structure
Abstract
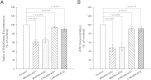
The inner mitochondrial membrane (IMM) invaginates to form cristae and the maintenance of cristae depends on the mitochondrial contact site (MICOS) complex. Mitofilin and CHCHD6, which physically interact, are two components of the MICOS. In this study, we performed immunoprecipitation experiments with Mitofilin and CHCHD6 antibodies and identified a complex containing Mitofilin, Sam50, and CHCHD 3 and 6. Using transcription activator-like effector nucleases (TALENs), we generated knockdown/knockout clones of Mitofilin and CHCHD6. Transmission electron microscopy (TEM) revealed that vesicle-like cristae morphology appeared in cell lines lacking Mitofilin, and mitochondria exhibited lower cristae density in CHCHD6-knockout cells. Immunoblot analysis showed that knockdown of Mitofilin, but not knockout of CHCHD6, affected their binding partners that control cristae morphology. We also demonstrated that Mitofilin and CHCHD6 directly interacted with Sam50. Additionally, we observed that Mitofilin-knockdown cells showed decreased mitochondrial membrane potential (ΔΨm) and intracellular ATP content, which were minimally affected in CHCHD6-knockout cells. Taken together, we conclude that the integrity of MICOS and its efficient interaction with Sam50 are indispensable for cristae organization, which is relevant to mitochondrial function.
Figures






References
-
- Perkins G. et al. Electron tomography of neuronal mitochondria: three-dimensional structure and organization of cristae and membrane contacts. J Struct Biol 119, 260–272 (1997). - PubMed
-
- Reichert A. S. & Neupert W. Mitochondriomics or what makes us breathe. Trends Genet 20, 555–562 (2004). - PubMed
-
- Zick M., Rabl R. & Reichert A. S. Cristae formation—linking ultrastructure and function of mitochondria. Bba-mol Cell Res 1793, 5–19 (2009). - PubMed
-
- Trimmer P. A. et al. Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines. Exp Neurol 162, 37–50 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

