Preclinical Evaluations To Identify Optimal Linezolid Regimens for Tuberculosis Therapy
- PMID: 26530386
- PMCID: PMC4631805
- DOI: 10.1128/mBio.01741-15
Preclinical Evaluations To Identify Optimal Linezolid Regimens for Tuberculosis Therapy
Abstract
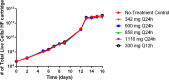
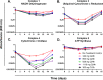
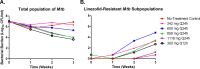
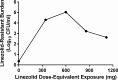
Linezolid is an oxazolidinone with potent activity against Mycobacterium tuberculosis. Linezolid toxicity in patients correlates with the dose and duration of therapy. These toxicities are attributable to the inhibition of mitochondrial protein synthesis. Clinically relevant linezolid regimens were simulated in the in vitro hollow-fiber infection model (HFIM) system to identify the linezolid therapies that minimize toxicity, maximize antibacterial activity, and prevent drug resistance. Linezolid inhibited mitochondrial proteins in an exposure-dependent manner, with toxicity being driven by trough concentrations. Once-daily linezolid killed M. tuberculosis in an exposure-dependent manner. Further, 300 mg linezolid given every 12 hours generated more bacterial kill but more toxicity than 600 mg linezolid given once daily. None of the regimens prevented linezolid resistance. These findings show that with linezolid monotherapy, a clear tradeoff exists between antibacterial activity and toxicity. By identifying the pharmacokinetic parameters linked with toxicity and antibacterial activity, these data can provide guidance for clinical trials evaluating linezolid in multidrug antituberculosis regimens.
Importance: The emergence and spread of multidrug-resistant M. tuberculosis are a major threat to global public health. Linezolid is an oxazolidinone that is licensed for human use and has demonstrated potent activity against multidrug-resistant M. tuberculosis. However, long-term use of linezolid has shown to be toxic in patients, often resulting in thrombocytopenia. We examined therapeutic linezolid regimens in an in vitro model to characterize the exposure-toxicity relationship. The antibacterial activity against M. tuberculosis was also assessed for these regimens, including the amplification or suppression of resistant mutant subpopulations by the chosen regimen. Higher exposures of linezolid resulted in greater antibacterial activity, but with more toxicity and, for some regimens, increased resistant mutant subpopulation amplification, illustrating the trade-off between activity and toxicity. These findings can provide valuable insight for designing optimal dosage regimens for linezolid that are part of the long combination courses used to treat multidrug-resistant M. tuberculosis.
Copyright © 2015 Brown et al.
Figures


References
-
- Aziz MA, Wright A, Laszlo A, De MA, Portaels F, Van DA, Wells C, Nunn P, Blanc L, Raviglione M, WHO/International Union Against Tuberculosis And Lung Disease Global Project on Anti-tuberculosis Drug Resistance Surveillance . 2006. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet 368:2142–2154. - PubMed
-
- Shah NS, Wright A, Bai G, Barrera L, Boulahbal F, Martín-Casabona N, Drobniewski F, Gilpin C, Havelková M, Lepe R, Lumb R, Metchock B, Portaels F, Rodrigues MF, Rüsch-Gerdes S, Van Deun A, Vincent V, Laserson K, Wells C, Cegielski JP. 2007. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis 13:380–387. doi:10.3201/eid1303.061400. - DOI - PMC - PubMed
-
- Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, Via LE, Goldfeder LC, Kang E, Jin B, Park H, Kwak H, Kim H, Jeon H, Jeong I, Joh JS, Chen RY, Olivier KN, Shaw PA, Follmann D, Song SD, Lee JK, Lee D, Kim CT, Dartois V, Park SK, Cho SN, Barry CE III. 2012. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 367:1508–1518. doi:10.1056/NEJMoa1201964. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
