Standardization, Evaluation, and Area-Under-Curve Analysis of Human and Murine Treg Suppressive Function
- PMID: 26530794
- PMCID: PMC5554116
- DOI: 10.1007/978-1-4939-3139-2_4
Standardization, Evaluation, and Area-Under-Curve Analysis of Human and Murine Treg Suppressive Function
Abstract
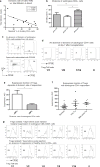
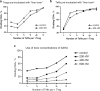
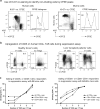
FOXP3+ T-regulatory (Treg) cells have important roles in immune homeostasis, and alterations in their number and function can predispose to diseases ranging from autoimmunity to allograft rejection and tumor growth. Reliable identification of human Tregs remains a persistent problem due to a lack of specific markers. The most definitive Treg characterization currently involves combined assessment of phenotypic, epigenetic and functional parameters, with the latter typically involving in vitro Treg suppression assays. Unfortunately, suppression assays are frequently performed using differing methods and readouts, limiting comparisons between studies. We provide a perspective on our experience with human and murine Treg suppression assay conditions, including Treg data obtained in clinical transplant studies, Tregs isolated from healthy donors and treated with epigenetically active compounds, and Tregs from standard murine strains (C57BL/6 and BALB/c). We provide detailed descriptions and illustrations of typical problems, shortcomings and troubleshooting; describe new modifications and approaches; and present a new method for calculation of suppressive assay data using a modified area-under-curve (AUC) method. This method allows us to directly compare Treg suppressive function between multiple patients (such as in clinical transplant studies), to reliably track changes in Treg function from the same person over time, or compare effects of Treg-modulating compounds tested with different healthy donors Tregs in separate or combined experimental settings.
Keywords: FOXP3+ regulatory T cells; Suppression assay; Tregs.
Figures






References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. - PubMed
-
- Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. - PubMed
-
- Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 DK087270/DK/NIDDK NIH HHS/United States
- R01 DK106243/DK/NIDDK NIH HHS/United States
- DK087270/DK/NIDDK NIH HHS/United States
- K08 DK092282/DK/NIDDK NIH HHS/United States
- K08AI095353/AI/NIAID NIH HHS/United States
- UL1-RR026314/RR/NCRR NIH HHS/United States
- K08DK092282/DK/NIDDK NIH HHS/United States
- P01 AI073489/AI/NIAID NIH HHS/United States
- K08 AI095353/AI/NIAID NIH HHS/United States
- UL1 TR001425/TR/NCATS NIH HHS/United States
- AI073489/AI/NIAID NIH HHS/United States
- UL1 RR026314/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

