A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results
- PMID: 26530985
- PMCID: PMC4632358
- DOI: 10.1186/s13063-015-1023-4
A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results
Abstract
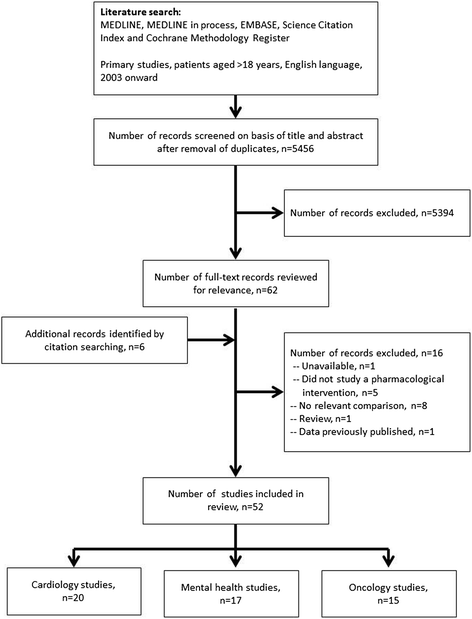
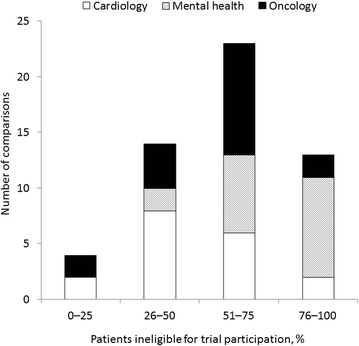
Randomized controlled trials (RCTs) are conducted under idealized and rigorously controlled conditions that may compromise their external validity. A literature review was conducted of published English language articles that reported the findings of studies assessing external validity by a comparison of the patient sample included in RCTs reporting on pharmaceutical interventions with patients from everyday clinical practice. The review focused on publications in the fields of cardiology, mental health, and oncology. A range of databases were interrogated (MEDLINE; EMBASE; Science Citation Index; Cochrane Methodology Register). Double-abstract review and data extraction were performed as per protocol specifications. Out of 5,456 de-duplicated abstracts, 52 studies met the inclusion criteria (cardiology, n = 20; mental health, n = 17; oncology, n = 15). Studies either performed an analysis of the baseline characteristics (demographic, socioeconomic, and clinical parameters) of RCT-enrolled patients compared with a real-world population, or assessed the proportion of real-world patients who would have been eligible for RCT inclusion following the application of RCT inclusion/exclusion criteria. Many of the included studies concluded that RCT samples are highly selected and have a lower risk profile than real-world populations, with the frequent exclusion of elderly patients and patients with co-morbidities. Calculation of ineligibility rates in individual studies showed that a high proportion of the general disease population was often excluded from trials. The majority of studies (n = 37 [71.2 %]) explicitly concluded that RCT samples were not broadly representative of real-world patients and that this may limit the external validity of the RCT. Authors made a number of recommendations to improve external validity. Findings from this review indicate that there is a need to improve the external validity of RCTs such that physicians treating patients in real-world settings have the appropriate evidence on which to base their clinical decisions. This goal could be achieved by trial design modification to include a more representative patient sample and by supplementing RCT evidence with data generated from observational studies. In general, a thoughtful approach to clinical evidence generation is required in which the trade-offs between internal and external validity are considered in a holistic and balanced manner.
Figures
Comment in
-
Disparity in the selection of patients in clinical trials.Lancet. 2022 Mar 12;399(10329):1048. doi: 10.1016/S0140-6736(22)00176-3. Lancet. 2022. PMID: 35279257 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

