Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents
- PMID: 26531048
- PMCID: PMC4632025
- DOI: 10.1038/srep16186
Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents
Abstract
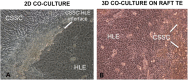
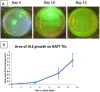
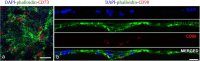
Human limbal epithelial cells (HLE) and corneal stromal stem cells (CSSC) reside in close proximity in vivo in the corneal limbal stem cell niche. However, HLE are typically cultured in vitro without supporting niche cells. Here, we re-create the cell-cell juxtaposition of the native environment in vitro, to provide a tool for investigation of epithelial-stromal cell interactions and to optimize HLE culture conditions for potential therapeutic application. RAFT (Real Architecture For 3D Tissue) tissue equivalents (TEs) were used as a 3-dimensional substrate for co-culturing HLE and CSSC. Our results demonstrate that a monolayer of HLE that maintained expression of p63α, ABCB5, CK8 and CK15 (HLE markers), formed on the surface of RAFT TEs within 13 days of culture. CSSC remained in close proximity to HLE and maintained expression of mesenchymal stem cell markers. This simple technique has a short preparation time of only 15 days with the onset of HLE layering and differentiation observed. Furthermore, co-cultivation of HLE with another niche cell type (CSSC) directly on RAFT TEs, eliminates the requirement for animal-derived feeder cells. RAFT TEs may be useful for future therapeutic delivery of multiple cell types to restore the limbal niche following ocular surface injury or disease.
Figures


References
-
- Schofield R. The relationship between the spleen colony forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978). - PubMed
-
- Davanger M. & Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229(5286), 560–561 (1971). - PubMed
-
- Dua H. S., Joseph A., Shanmuganathan V. A. & Jones R. E. Stem cell differentiation and the effects of deficiency. Eye 17, 877–885 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

