The A673T mutation in the amyloid precursor protein reduces the production of β-amyloid protein from its β-carboxyl terminal fragment in cells
- PMID: 26531305
- PMCID: PMC4632685
- DOI: 10.1186/s40478-015-0247-6
The A673T mutation in the amyloid precursor protein reduces the production of β-amyloid protein from its β-carboxyl terminal fragment in cells
Abstract
Introduction: The A673T mutation in the amyloid precursor protein (APP) protects against Alzheimer's disease by reducing β-amyloid protein (Aβ) production. This mutation reduced the release of the soluble APP fragment (sAPPβ), which is processed by β-secretase, suggesting a concomitant decrease in the β-carboxyl fragment of APP (C99), which is a direct substrate of γ-secretase for Aβ production. However, it remains controversial whether the level of C99 is significantly reduced in cells expressing APP that carry A673T as the cause of reduced Aβ production. Here, we investigated the effect of the A673T mutation in C99 on γ-cleavage in cells.
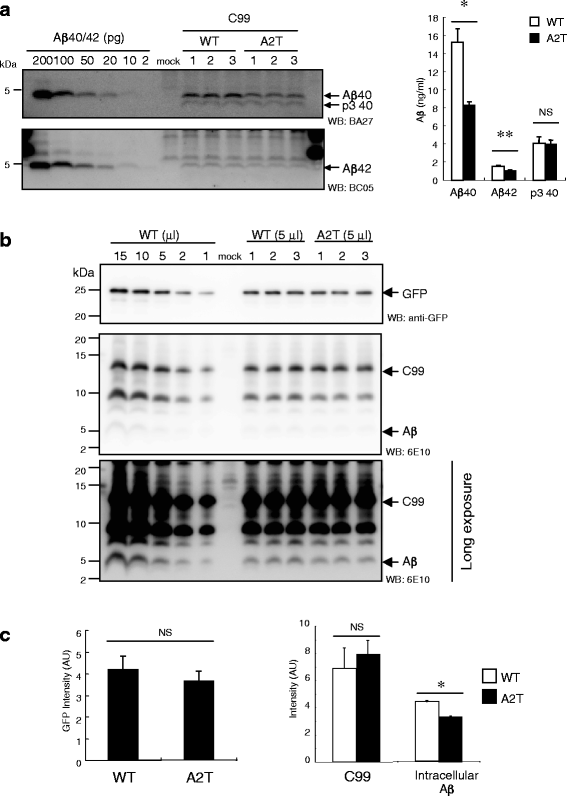
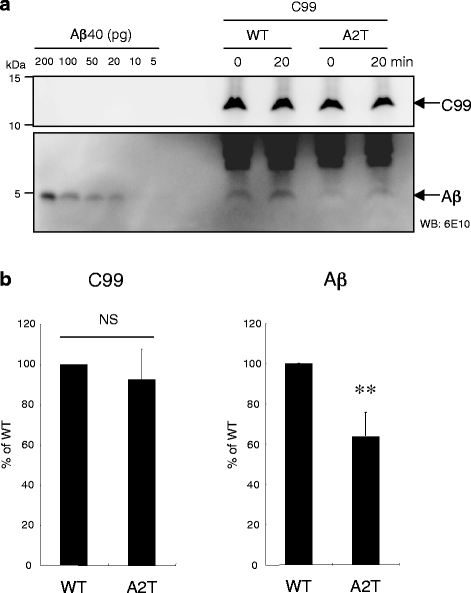
Results: We found that the level of C99 in cells expressing APP A673T was indistinctive of that observed in cells expressing wild-type APP, although the release of sAPPβ was significantly reduced in the APP A673T cells. In addition, our reconstituted β-secretase assay demonstrated no significant difference in β-cleavage on an APP fragment carrying the A673T mutation compared with the wild-type fragment. Importantly, cells expressing C99 containing the A673T mutation (C99 A2T; in accordance with the Aβ numbering) produced roughly half the level of Aβ compared with the wild-type C99, suggesting that the C99 A2T is an insufficient substrate of γ-secretase in cells. A cell-free γ-secretase assay revealed that Aβ production from the microsomal fraction of cells expressing C99 A2T was diminished. A sucrose gradient centrifugation analysis indicated that the levels of the C99 A2T that was codistributed with γ-secretase components in the raft fractions were reduced significantly.
Conclusions: Our data indicate that the A673T mutation in APP alters the release of sAPPβ, but not the C99 level, and that the C99 A2T is an inefficient substrate for γ-secretase in cell-based assay.
Figures





References
-
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–41. doi: 10.1126/science.286.5440.735. - DOI - PubMed
-
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

