Excess BMP Signaling in Heterotopic Cartilage Forming in Prg4-null TMJ Discs
- PMID: 26534931
- PMCID: PMC4766954
- DOI: 10.1177/0022034515613508
Excess BMP Signaling in Heterotopic Cartilage Forming in Prg4-null TMJ Discs
Abstract
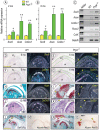
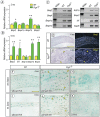
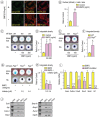
Heterotopic cartilage develops in certain pathologic conditions, including those affecting the human temporomandibular joint (TMJ), but the underlying molecular mechanisms remain obscure. This is in part due to the fact that a reliable animal model of such TMJ diseases is not available. Here, we show that aberrant chondrocyte differentiation and ectopic cartilage formation occur spontaneously in proteoglycan 4 (Prg4) mutant TMJ discs without further invasive procedure. By 2 mo of age, mutant disc cells displayed chondrocyte transdifferentiation, accompanied by strong expression of cartilage master gene Sox9 and matrix genes aggrecan and type II collagen. By 6 mo, heterotopic cartilage had formed in the discs and expressed cartilage hypertrophic markers Runx2 and ColX. The ectopic tissue grew in size over time and exhibited regional mineralization by 12 mo. Bone morphogenetic protein (BMP) signaling was activated with the ectopic chondrogenic cells and chondrocytes, as indicated by phosphorylated Smad 1/5/8 nuclear staining and by elevated expression of Bmp2, Bmpr1b, Bmpr2, and BMP signaling target genes. Likewise, we found that upon treatment with recombinant human BMP 2 in high-density micromass culture, mutant disc cells differentiated into chondrocytes and synthesized cartilage matrix more robustly than control cells. Importantly, a specific kinase inhibitor of BMP receptors drastically attenuated chondrogenesis in recombinant human BMP 2-treated mutant disc cultures. Unexpectedly, we found that Prg4 was expressed at joint-associated sites, including disc/muscle insertion and muscle/bone interface, and all these structures were abnormal in Prg4 mutants. Our data indicate that Prg4 is needed for TMJ disc integrity and function and that its absence leads to ectopic chondrogenesis and cartilage formation in conjunction with abnormal BMP signaling. Our findings imply that the BMP signaling pathway could be a potential therapeutic target for prevention or inhibition of ectopic cartilage formation in TMJ disease.
Keywords: TMJ disorder; articular disc; ectopic cartilage; joint lubrication; lubricin; temporomandibular joint.
© International & American Associations for Dental Research 2015.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures


References
-
- Asai S, Otsuru S, Candela ME, Cantley L, Uchibe K, Hofmann TJ, Zhang K, Wapner KL, Soslowsky LJ, Horwitz EM, et al. 2014. Tendon progenitor cells in injured tendons have strong chondrogenic potential: the CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells. 32(12):3266–3277. - PMC - PubMed
-
- Bahabri SA, Suwairi WM, Laxer RM, Polinkovsky A, Dalaan AA, Warman ML. 1998. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum. 41(4):730–735. - PubMed
-
- Berkovitz BK, Pacy J. 2000. Age changes in the cells of the intra-articular disc of the temporomandibular joints of rats and marmosets. Arch Oral Biol. 45(11):987–995. - PubMed
-
- Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. 2008. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res. 23(3):305–313. - PMC - PubMed
-
- Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. 1998. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 142(1):295–305. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

