Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors
- PMID: 26534966
- PMCID: PMC5027962
- DOI: 10.1158/1078-0432.CCR-15-0491
Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors
Abstract
Purpose: Ipilimumab is a first-in-class immune checkpoint inhibitor approved for treatment of metastatic melanoma but not studied in children until this phase I protocol.
Experimental design: This study examined safety, pharmacokinetics, and immunogenicity, and immune correlates of ipilimumab administered to subjects ≤21 years old with recurrent or progressive solid tumors. Dose escalation cohorts received 1, 3, 5, or 10 mg/m(2) intravenously every 3 weeks in a 3 + 3 design. Response was assessed after 6 weeks and 12 weeks, and then every 3 months. Treatment was continued until disease progression or unacceptable toxicity.
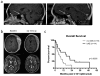
Results: Thirty-three patients received 72 doses of ipilimumab. Patients enrolled had melanoma (n = 12), sarcoma (n = 17), or other refractory solid tumors (n = 4). Immune-related adverse events included pancreatitis, pneumonitis, colitis, endocrinopathies, and transaminitis with dose-limiting toxicities observed at 5 and 10 mg/kg dose levels. Pharmacokinetics revealed a half-life of 8 to 15 days. At day 21, subjects had increased levels of cycling T cells, but no change in regulatory T-cell populations. Six subjects had confirmed stable disease for 4 to 10 cycles (melanoma, osteosarcoma, clear cell sarcoma, and synovial sarcoma).
Conclusions: Ipilimumab was safely administered to pediatric patients using management algorithms for immune-related toxicities. The spectrum of immune-related adverse events is similar to those described in adults; however, many of the pediatric toxicities were evident after a single dose. Although no objective tumor regressions were observed with ipilimumab as a single agent, subjects with immune-related toxicities had an increased overall survival compared with those who showed no evidence of breaking tolerance.
©2015 American Association for Cancer Research.
Figures

References
-
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. - PubMed
-
- Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5275–83. - PubMed
-
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. The Lancet Oncology. 2010;11:155–64. - PubMed

