Treatment Response Assessment in IDH-Mutant Glioma Patients by Noninvasive 3D Functional Spectroscopic Mapping of 2-Hydroxyglutarate
- PMID: 26534967
- PMCID: PMC4818725
- DOI: 10.1158/1078-0432.CCR-15-0656
Treatment Response Assessment in IDH-Mutant Glioma Patients by Noninvasive 3D Functional Spectroscopic Mapping of 2-Hydroxyglutarate
Abstract
Purpose: Measurements of objective response rates are critical to evaluate new glioma therapies. The hallmark metabolic alteration in gliomas with mutant isocitrate dehydrogenase (IDH) is the overproduction of oncometabolite 2-hydroxyglutarate (2HG), which plays a key role in malignant transformation. 2HG represents an ideal biomarker to probe treatment response in IDH-mutant glioma patients, and we hypothesized a decrease in 2HG levels would be measureable by in vivo magnetic resonance spectroscopy (MRS) as a result of antitumor therapy.
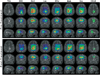
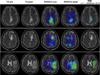
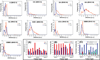
Experimental design: We report a prospective longitudinal imaging study performed in 25 IDH-mutant glioma patients receiving adjuvant radiation and chemotherapy. A newly developed 3D MRS imaging was used to noninvasively image 2HG. Paired Student t test was used to compare pre- and posttreatment tumor 2HG values. Test-retest measurements were performed to determine the threshold for 2HG functional spectroscopic maps (fSM). Univariate and multivariate regression were performed to correlate 2HG changes with Karnofsky performance score (KPS).
Results: We found that mean 2HG (2HG/Cre) levels decreased significantly (median = 48.1%; 95% confidence interval = 27.3%-56.5%;P= 0.007) in the posttreatment scan. The volume of decreased 2HG correlates (R(2)= 0.88,P= 0.002) with clinical status evaluated by KPS.
Conclusions: We demonstrate that dynamic measurements of 2HG are feasible by 3D fSM, and the decrease of 2HG levels can monitor treatment response in patients with IDH-mutant gliomas. Our results indicate that quantitative in vivo 2HG imaging may be used for precision medicine and early response assessment in clinical trials of therapies targeting IDH-mutant gliomas.
©2015 American Association for Cancer Research.
Conflict of interest statement
Figures

References
-
- van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–990. - PubMed
-
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. - PubMed
-
- van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJ, Jaeckle K, Junck L, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12:583–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 EB015894/EB/NIBIB NIH HHS/United States
- P41 RR008079/RR/NCRR NIH HHS/United States
- S10 RR021110/RR/NCRR NIH HHS/United States
- L30 CA117071/CA/NCI NIH HHS/United States
- P30 NS057091/NS/NINDS NIH HHS/United States
- K24CA125440A/CA/NCI NIH HHS/United States
- NIH R01CA129371/CA/NCI NIH HHS/United States
- NIH S10RR013026/RR/NCRR NIH HHS/United States
- R01 CA129371/CA/NCI NIH HHS/United States
- S10RR023401/RR/NCRR NIH HHS/United States
- K24 CA125440/CA/NCI NIH HHS/United States
- K22 CA178269/CA/NCI NIH HHS/United States
- S10RR021110/RR/NCRR NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- 1K22CA178269-01/CA/NCI NIH HHS/United States
- P50 CA165962/CA/NCI NIH HHS/United States
- S10 RR023401/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

