Establishing Good Practices for Exposure-Response Analysis of Clinical Endpoints in Drug Development
- PMID: 26535157
- PMCID: PMC4625861
- DOI: 10.1002/psp4.12015
Establishing Good Practices for Exposure-Response Analysis of Clinical Endpoints in Drug Development
Abstract
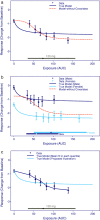
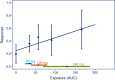
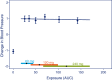
This tutorial aims at promoting good practices for exposure-response (E-R) analyses of clinical endpoints in drug development. The focus is on practical aspects of E-R analyses to assist modeling scientists with a process of performing such analyses in a consistent manner across individuals and projects and tailored to typical clinical drug development decisions. This includes general considerations for planning, conducting, and visualizing E-R analyses, and how these are linked to key questions.
Figures

References
-
- Pinheiro J. Duffull S. Exposure response—getting the dose right. Pharmaceut. Statist. 2009;8:173–175. & ) - PubMed
-
- Ette EI. Williams PJ. Population pharmacokinetics I: background, concepts, and models. Ann. Pharmacother. 2004;38:1702–1706. & ) - PubMed
-
- Ette EI. Williams PJ. Population pharmacokinetics II: estimation methods. Ann. Pharmacother. 2004;38:1907–1915. & ) - PubMed
-
- Ette EI. Williams PJ. Population pharmacokinetics III: design, analysis, and application of population pharmacokinetic studies. Ann. Pharmacother. 2004;38:2136–2144. & ) - PubMed
-
- Sherwin CMT, Kiang TKL, Spigarelli MG. Ensom MHH. Fundamentals of population pharmacokinetic modeling, validation methods. Clin. Pharmacokinet. 2012;51:573–590. & ) - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

