(E)-4-(3,4-Dimethoxyphenyl)but-3-en-1-ol Enhances Melanogenesis through Increasing Upstream Stimulating Factor-1-Mediated Tyrosinase Expression
- PMID: 26535571
- PMCID: PMC4633108
- DOI: 10.1371/journal.pone.0141988
(E)-4-(3,4-Dimethoxyphenyl)but-3-en-1-ol Enhances Melanogenesis through Increasing Upstream Stimulating Factor-1-Mediated Tyrosinase Expression
Abstract
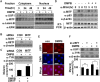
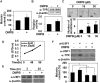
We investigated the potential melanogenic effect of compounds from Zingiber cassumunar Roxb. Our data revealed that chloroform-soluble extract of Z. cassumunar enhanced melanin synthesis in B16F10 melanoma cells. Among the components of the chloroform extract, (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-ol (DMPB) increased melanogenesis in both B16F10 cells and human primary melanocytes. In B16F10 cells, DMPB enhanced the activation of ERK and p38, and the level of tyrosinase. Although the level of microphthalmia-associated transcription factor was unchanged in DMPB-treated B16F10 cells, DMPB increased levels and nuclear localization of upstream stimulating factor-1 (USF1). Consistently, DMPB-mediated melanin synthesis was diminished in USF1-knockdown cells. Furthermore, DMPB induced hyperpigmentation in brown guinea pigs in vivo. Together, these data suggest that DMPB may promote melanin synthesis via USF1 dependent fashion and could be used as a clinical therapeutic agent against hypopigmentation-associated diseases.
Conflict of interest statement
Figures




References
-
- Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, et al. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006; 20: 1486–1488. - PubMed
-
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004; 84: 1155–1228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

