Ocular Hypotensive Effects of the ATP-Sensitive Potassium Channel Opener Cromakalim in Human and Murine Experimental Model Systems
- PMID: 26535899
- PMCID: PMC4633217
- DOI: 10.1371/journal.pone.0141783
Ocular Hypotensive Effects of the ATP-Sensitive Potassium Channel Opener Cromakalim in Human and Murine Experimental Model Systems
Erratum in
-
Correction: Ocular Hypotensive Effects of the ATP-Sensitive Potassium Channel Opener Cromakalim in Human and Murine Experimental Model Systems.PLoS One. 2015 Dec 15;10(12):e0144791. doi: 10.1371/journal.pone.0144791. eCollection 2015. PLoS One. 2015. PMID: 26669304 Free PMC article. No abstract available.
Abstract
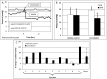
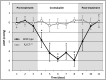
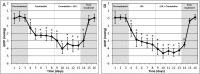
Elevated intraocular pressure (IOP) is the most prevalent and only treatable risk factor for glaucoma, a leading cause of irreversible blindness worldwide. Unfortunately, all current therapeutics used to treat elevated IOP and glaucoma have significant and sometimes irreversible side effects necessitating the development of novel compounds. We evaluated the IOP lowering ability of the broad spectrum KATP channel opener cromakalim. Cultured human anterior segments when treated with 2 μM cromakalim showed a decrease in pressure (19.33 ± 2.78 mmHg at 0 hours to 13.22 ± 2.64 mmHg at 24 hours; p<0.001) when compared to vehicle treated controls (15.89 ± 5.33 mmHg at 0 h to 15.56 ± 4.88 mmHg at 24 hours; p = 0.89). In wild-type C57BL/6 mice, cromakalim reduced IOP by 18.75 ± 2.22% compared to vehicle treated contralateral eyes (17.01 ± 0.32 mmHg at 0 hours to 13.82 ± 0.37 mmHg at 24 hours; n = 10, p = 0.002). Cromakalim demonstrated an additive effect when used in conjunction with latanoprost free acid, a common ocular hypotensive drug prescribed to patients with elevated IOP. To examine KATP channel subunit specificity, Kir6.2(-/-) mice were treated with cromakalim, but unlike wild-type animals, no change in IOP was noted. Histologic analysis of treated and control eyes in cultured human anterior segments and in mice showed similar cell numbers and extracellular matrix integrity within the trabecular meshwork, with no disruptions in the inner and outer walls of Schlemm's canal. Together, these studies suggest that cromakalim is a potent ocular hypotensive agent that lowers IOP via activation of Kir6.2 containing KATP channels, its effect is additive when used in combination with the commonly used glaucoma drug latanoprost, and is not toxic to cells and tissues of the aqueous humor outflow pathway, making it a candidate for future therapeutic development.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

