Loss of Metabotropic Glutamate Receptor 5 Function on Peripheral Benzodiazepine Receptor in Mice Prenatally Exposed to LPS
- PMID: 26536027
- PMCID: PMC4633140
- DOI: 10.1371/journal.pone.0142093
Loss of Metabotropic Glutamate Receptor 5 Function on Peripheral Benzodiazepine Receptor in Mice Prenatally Exposed to LPS
Abstract
Parental microglial induced neuroinflammation, triggered by bacterial- or viral infections, can induce neuropsychiatric disorders like schizophrenia and autism to offspring in animal models. Recent investigations suggest that microglia, the resident immune cells of the brain, provides a link between neurotransmission, immune cell activation, brain inflammation and neuronal dysfunction seen with the offspring. Relatively little is known about how reduction of brain inflammation and restoration of glial function are associated with diminution of brain degeneration and behavioral deficits in offspring. Increased mGluR5 expression and the long-lasting excitotoxic effects of the neurotoxin during brain development are associated with the glial dysfunctions. We investigated the relationship of mGluR5 and PBR and how they regulate glial function and inflammatory processes in mice prenatally exposed to LPS (120μg/kg, between gestational days 15 and 17), an inflammatory model of a psychiatric disorder. Using PET imaging, we showed that pharmacological activation of mGluR5 during 5 weeks reduced expression of classic inflammation marker PBR in many brain areas and that this molecular association was not present in LPS-exposed offspring. The post-mortem analysis revealed that the down regulation of PBR was mediated through activation of mGluR5 in astrocytes. In addition, we demonstrated that this interaction is defective in a mouse model of the psychiatric deficit offering a novel insight of mGluR5 involvement to brain related disorders and PBR related imaging studies. In conclusion, mGluR5 driven glutamatergic activity regulates astrocytic functions associated with PBR (cholesterol transport, neurosteroidogenesis, glial phenotype) during maturation and could be associated with neuropsychiatric disorders in offspring.
Conflict of interest statement
Figures




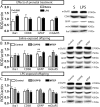
References
-
- Pellegrino D, Cicchetti F, Wang X, Zhu A, Yu M, Saint-Pierre M, et al. (2007) Modulation of dopaminergic and glutamatergic brain function: PET studies on parkinsonian rats. J Nucl Med 48: 1147–1153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

