Broadening of Virus-Specific CD8+ T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PMID: 26536034
- PMCID: PMC4633064
- DOI: 10.1371/journal.ppat.1005247
Broadening of Virus-Specific CD8+ T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Abstract
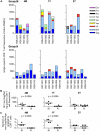
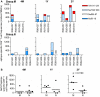
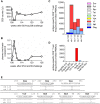
Control of HIV replication is a rare immunological event, providing clues to understand the viral control mechanism. CD8+ T-cell responses are crucial for virus control, but it is unclear whether lasting HIV containment can be achieved after establishment of infection. Here, we describe lasting SIV containment in a macaque AIDS model. Analysis of ten rhesus macaques that controlled viremia for 2 years post-infection found accumulation of proviral gag and nef CD8+ T-cell escape mutations in four of them. These four controllers mounted CD8+ T cells targeting Gag, Nef, and other viral proteins at 4 months, suggesting that broadening of CD8+ T-cell targets can be an indicator of the beginning of viral control failure. The remaining six aviremic SIV controllers, however, harbored proviruses without mutations and showed no or little broadening of their CD8+ T-cell responses in the chronic phase. Indeed, three of the latter six exhibiting no change in CD8+ T-cell targets showed gradual decreases in SIV-specific CD8+ T-cell frequencies, implying a concomitant reduction in viral replication. Thus, stability of the breadth of virus-specific CD8+ T-cell responses may represent a status of lasting HIV containment by CD8+ T cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Rosenberg E.S., Altfeld M., Poon S.H., Phillips M.N., Wilkes B.M., Eldridge R.L., Robbins G.K., D'Aquila R.T., Goulder P.J., and Walker B.D. (2000). Immune control of HIV-1 after early treatment of acute infection. Nature 407, 523–526. - PubMed
-
- Hocqueloux L., Prazuck T., Avettand-Fenoel V., Lafeuillade A., Cardon B., Viard J.P., and Rouzioux C. (2010). Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS 24, 1598–1601. - PubMed
-
- Saez-Cirion A., Bacchus C., Hocqueloux L., Avettand-Fenoel V., Girault I., Lecuroux C., Potard V., Versmisse P., Melard A., Prazuck T., et al. (2013). Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9, e1003211 10.1371/journal.ppat.1003211 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

