Basomedial amygdala mediates top-down control of anxiety and fear
- PMID: 26536109
- PMCID: PMC4780260
- DOI: 10.1038/nature15698
Basomedial amygdala mediates top-down control of anxiety and fear
Abstract
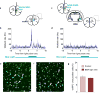
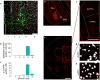
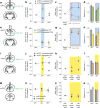
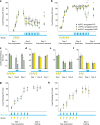
Anxiety-related conditions are among the most difficult neuropsychiatric diseases to treat pharmacologically, but respond to cognitive therapies. There has therefore been interest in identifying relevant top-down pathways from cognitive control regions in medial prefrontal cortex (mPFC). Identification of such pathways could contribute to our understanding of the cognitive regulation of affect, and provide pathways for intervention. Previous studies have suggested that dorsal and ventral mPFC subregions exert opposing effects on fear, as do subregions of other structures. However, precise causal targets for top-down connections among these diverse possibilities have not been established. Here we show that the basomedial amygdala (BMA) represents the major target of ventral mPFC in amygdala in mice. Moreover, BMA neurons differentiate safe and aversive environments, and BMA activation decreases fear-related freezing and high-anxiety states. Lastly, we show that the ventral mPFC-BMA projection implements top-down control of anxiety state and learned freezing, both at baseline and in stress-induced anxiety, defining a broadly relevant new top-down behavioural regulation pathway.
Conflict of interest statement
The authors declare no competing financial interests.
Figures











References
-
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

