Anaplastic Lymphoma Kinase Acts in the Drosophila Mushroom Body to Negatively Regulate Sleep
- PMID: 26536237
- PMCID: PMC4633181
- DOI: 10.1371/journal.pgen.1005611
Anaplastic Lymphoma Kinase Acts in the Drosophila Mushroom Body to Negatively Regulate Sleep
Abstract
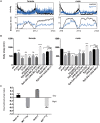
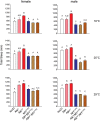
Though evidence is mounting that a major function of sleep is to maintain brain plasticity and consolidate memory, little is known about the molecular pathways by which learning and sleep processes intercept. Anaplastic lymphoma kinase (Alk), the gene encoding a tyrosine receptor kinase whose inadvertent activation is the cause of many cancers, is implicated in synapse formation and cognitive functions. In particular, Alk genetically interacts with Neurofibromatosis 1 (Nf1) to regulate growth and associative learning in flies. We show that Alk mutants have increased sleep. Using a targeted RNAi screen we localized the negative effects of Alk on sleep to the mushroom body, a structure important for both sleep and memory. We also report that mutations in Nf1 produce a sexually dimorphic short sleep phenotype, and suppress the long sleep phenotype of Alk. Thus Alk and Nf1 interact in both learning and sleep regulation, highlighting a common pathway in these two processes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

