Acceleration of reaction in charged microdroplets
- PMID: 26537403
- PMCID: PMC5446060
- DOI: 10.1017/S0033583515000086
Acceleration of reaction in charged microdroplets
Abstract
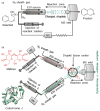
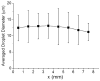
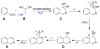
Using high-resolution mass spectrometry, we have studied the synthesis of isoquinoline in a charged electrospray droplet and the complexation between cytochrome c and maltose in a fused droplet to investigate the feasibility of droplets to drive reactions (both covalent and noncovalent interactions) at a faster rate than that observed in conventional bulk solution. In both the cases we found marked acceleration of reaction, by a factor of a million or more in the former and a factor of a thousand or more in the latter. We believe that carrying out reactions in microdroplets (about 1-15 μm in diameter corresponding to 0·5 pl - 2 nl) is a general method for increasing reaction rates. The mechanism is not presently established but droplet evaporation and droplet confinement of reagents appear to be two important factors among others. In the case of fused water droplets, evaporation has been shown to be almost negligible during the flight time from where droplet fusion occurs and the droplets enter the heated capillary inlet of the mass spectrometer. This suggests that (1) evaporation is not responsible for the acceleration process in aqueous droplet fusion and (2) the droplet-air interface may play a significant role in accelerating the reaction. We argue that this 'microdroplet chemistry' could be a remarkable alternative to accelerate slow and difficult reactions, and in conjunction with mass spectrometry, it may provide a new arena to study chemical and biochemical reactions in a confined environment.
Keywords: Electospray ionization; cytochrome c; droplet fusion; isoquinoline; kinetics; maltose.
Figures



References
-
- Badu-Tawiah A, Campbell D, Cooks RG. Accelerated C-N bond formation in dropcast thin films on ambient surfaces. Journal of the American Society for Mass Spectrometry. 2012;23(9):1461–1468. - PubMed
-
- Banerjee S. Induction of protein conformational change inside the charged electrospray droplet. Journal of Mass Spectrometry. 2013;48(2):193–204. - PubMed
-
- Banerjee S, Prakash H, Mazumdar S. Evidence of molecular fragmentation inside the charged droplets produced by electrospray process. Journal of the American Society for Mass Spectrometry. 2011;22(10):1707–1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

