Spermatogenesis: The Commitment to Meiosis
- PMID: 26537427
- PMCID: PMC4698398
- DOI: 10.1152/physrev.00013.2015
Spermatogenesis: The Commitment to Meiosis
Abstract
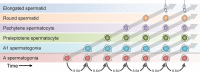
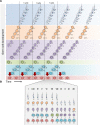
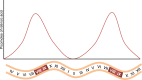
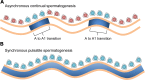
Mammalian spermatogenesis requires a stem cell pool, a period of amplification of cell numbers, the completion of reduction division to haploid cells (meiosis), and the morphological transformation of the haploid cells into spermatozoa (spermiogenesis). The net result of these processes is the production of massive numbers of spermatozoa over the reproductive lifetime of the animal. One study that utilized homogenization-resistant spermatids as the standard determined that human daily sperm production (dsp) was at 45 million per day per testis (60). For each human that means ∼1,000 sperm are produced per second. A key to this level of gamete production is the organization and architecture of the mammalian testes that results in continuous sperm production. The seemingly complex repetitious relationship of cells termed the "cycle of the seminiferous epithelium" is driven by the continuous commitment of undifferentiated spermatogonia to meiosis and the period of time required to form spermatozoa. This commitment termed the A to A1 transition requires the action of retinoic acid (RA) on the undifferentiated spermatogonia or prospermatogonia. In stages VII to IX of the cycle of the seminiferous epithelium, Sertoli cells and germ cells are influenced by pulses of RA. These pulses of RA move along the seminiferous tubules coincident with the spermatogenic wave, presumably undergoing constant synthesis and degradation. The RA pulse then serves as a trigger to commit undifferentiated progenitor cells to the rigidly timed pathway into meiosis and spermatid differentiation.
Copyright © 2016 the American Physiological Society.
Figures




References
-
- Amann R. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl 29: 469–487, 2008. - PubMed
-
- Ballow D, Meistrich ML, Matzuk M, Rajkovic A. Sohlh1 is essential for spermatogonial differentiation. Dev Biol 294: 161–167, 2006. - PubMed
-
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 38: 1430–1434, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

