Membrane fusion in muscle development and repair
- PMID: 26537430
- PMCID: PMC4679555
- DOI: 10.1016/j.semcdb.2015.10.026
Membrane fusion in muscle development and repair
Abstract
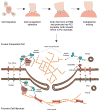
Mature skeletal muscle forms from the fusion of skeletal muscle precursor cells, myoblasts. Myoblasts fuse to other myoblasts to generate multinucleate myotubes during myogenesis, and myoblasts also fuse to other myotubes during muscle growth and repair. Proteins within myoblasts and myotubes regulate complex processes such as elongation, migration, cell adherence, cytoskeletal reorganization, membrane coalescence, and ultimately fusion. Recent studies have identified cell surface proteins, intracellular proteins, and extracellular signaling molecules required for the proper fusion of muscle. Many proteins that actively participate in myoblast fusion also coordinate membrane repair. Here we will review mammalian membrane fusion with specific attention to proteins that mediate myoblast fusion and muscle repair.
Keywords: Development; Fusion; Membrane; Muscle; Myoblast; Repair.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest
There are no conflicts of interest.
Figures

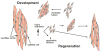

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

