Supervised pharmacy student-led medication review in primary care for patients with type 2 diabetes: a randomised controlled pilot study
- PMID: 26537500
- PMCID: PMC4636620
- DOI: 10.1136/bmjopen-2015-009246
Supervised pharmacy student-led medication review in primary care for patients with type 2 diabetes: a randomised controlled pilot study
Abstract
Objective: To pilot and feasibility-test supervised final year undergraduate pharmacy student-led medication reviews for patients with diabetes to enable definitive trial design.
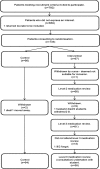
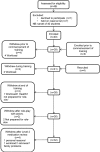
Method: Third year pharmacy students were recruited from one UK School of Pharmacy and trained to review patient's medical records and provide face-to-face consultations under supervision while situated within the patient's medical practice. Patients with type 2 diabetes were recruited by postal invitation letter from their medical practice and randomised via automated system to intervention or usual care. Diabetes-related clinical data, quality of life, patient reported beliefs, adherence and satisfaction with medicines information were collected with validated tools at baseline and 6 months postintervention. The process for collecting resource utilisation data was tested. Stakeholder meetings were held before and after intervention to develop study design and learn from its implementation. Recruitment and attrition rates were determined plus the quality of the outcome data. Power calculations for a definitive trial were performed on the different outcome measures to identify the most appropriate primary outcome measure.
Results: 792 patients were identified as eligible from five medical practices. 133 (16.8%) were recruited and randomised to control (n=66) or usual care (n=67). 32 students provided the complete intervention to 58 patients. Initial data analysis showed potential for impact in the right direction for some outcomes measured including glycated haemoglobin, quality of life and patient satisfaction with information about medicines. The intervention was found to be feasible and acceptable to patients. The pilot and feasibility study enabled the design of a future full randomised controlled trial.
Conclusions: Student and patient recruitment are possible. The intervention was well received and demonstrated some potential benefits. While the intervention was relatively inexpensive and provided an experiential learning opportunity for pharmacy students, its cost-effectiveness remains to be determined.
Trial registration number: ISRCTN26445805; Results.
Keywords: EDUCATION & TRAINING (see Medical Education & Training).
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

References
-
- Patient Safety Observatory. Safety in doses: medication safety incidents in the NHS. Secondary Safety in doses: medication safety incidents in the NHS 2007. http://www.nrls.npsa.nhs.uk/patient-safety-data/
-
- Barber N, Parsons J, Clifford S et al. . Patients’ problems with new medication for chronic conditions. Qual Saf Health Care 2004;13:172–5. http://qualitysafety.bmj.com/content/13/3/172.full.pdf+html?sid=eab8a148... (accessed 1 Dec 2013). 10.1136/qhc.13.3.172 - DOI - PMC - PubMed
-
- Taskforce on Medicines Partnership National Collaborative Medicines Management Services Programme. Room for review. A guide to medication review: the agenda for patients, practitioners and managers. London: Medicines Partnership, 2002.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
