Mitochondrial dynamics, mitophagy and cardiovascular disease
- PMID: 26537557
- PMCID: PMC5341713
- DOI: 10.1113/JP271301
Mitochondrial dynamics, mitophagy and cardiovascular disease
Abstract
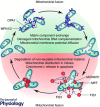
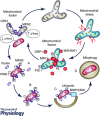
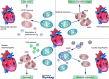
Cardiac hypertrophy is often initiated as an adaptive response to haemodynamic stress or myocardial injury, and allows the heart to meet an increased demand for oxygen. Although initially beneficial, hypertrophy can ultimately contribute to the progression of cardiac disease, leading to an increase in interstitial fibrosis and a decrease in ventricular function. Metabolic changes have emerged as key mechanisms involved in the development and progression of pathological remodelling. As the myocardium is a highly oxidative tissue, mitochondria play a central role in maintaining optimal performance of the heart. 'Mitochondrial dynamics', the processes of mitochondrial fusion, fission, biogenesis and mitophagy that determine mitochondrial morphology, quality and abundance have recently been implicated in cardiovascular disease. Studies link mitochondrial dynamics to the balance between energy demand and nutrient supply, suggesting that changes in mitochondrial morphology may act as a mechanism for bioenergetic adaptation during cardiac pathological remodelling. Another critical function of mitochondrial dynamics is the removal of damaged and dysfunctional mitochondria through mitophagy, which is dependent on the fission/fusion cycle. In this article, we discuss the latest findings regarding the impact of mitochondrial dynamics and mitophagy on the development and progression of cardiovascular pathologies, including diabetic cardiomyopathy, atherosclerosis, damage from ischaemia-reperfusion, cardiac hypertrophy and decompensated heart failure. We will address the ability of mitochondrial fusion and fission to impact all cell types within the myocardium, including cardiac myocytes, cardiac fibroblasts and vascular smooth muscle cells. Finally, we will discuss how these findings can be applied to improve the treatment and prevention of cardiovascular diseases.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures

References
-
- Abouhamed M, Reichenberg S, Robenek H & Plenz G (2003). Tropomyosin 4 expression is enhanced in dedifferentiating smooth muscle cells in vitro and during atherogenesis. Eur J Cell Biol 82, 473–482. - PubMed
-
- Anan R, Nakagawa M, Miyata M, Higuchi I, Nakao S, Suehara M, Osame M & Tanaka H (1995). Cardiac involvement in mitochondrial diseases: A study on 17 patients with documented mitochondrial DNA defects. Circulation 91, 955–961. - PubMed
-
- Ashrafian H , Docherty L, Leo V, Towlson C, Neilan M, Steeples V, Lygate CA, Hough T, Townsend S, Williams D, Wells S, Norris D, Glyn‐Jones S, Land J, Barbaric I, Lalanne Z, Denny P, Szumska D, Bhattacharya S, Griffin JL, Hargreaves I, Fernandez‐Fuentes N, Cheeseman M, Watkins H & Dear TN (2010). A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet 6, e1001000. - PMC - PubMed
-
- Ballinger SW, Patterson C, Knight‐Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K & Runge MS (2002). Mitochondrial integrity and function in atherogenesis. Circulation 106, 544–549. - PubMed
-
- Barry SP, Davidson SM & Townsend PA (2008). Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol 40, 2023–2039. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

