Application of Nanotrap technology for high sensitivity measurement of urinary outer surface protein A carboxyl-terminus domain in early stage Lyme borreliosis
- PMID: 26537892
- PMCID: PMC4634744
- DOI: 10.1186/s12967-015-0701-z
Application of Nanotrap technology for high sensitivity measurement of urinary outer surface protein A carboxyl-terminus domain in early stage Lyme borreliosis
Abstract
Objectives: Prompt antibiotic treatment of early stage Lyme borreliosis (LB) prevents progression to severe multisystem disease. There is a clinical need to improve the diagnostic specificity of early stage Lyme assays in the period prior to the mounting of a robust serology response. Using a novel analyte harvesting nanotechnology, Nanotrap particles, we evaluated urinary Borrelia Outer surface protein A (OspA) C-terminus peptide in early stage LB before and after treatment, and in patients suspected of late stage disseminated LB.
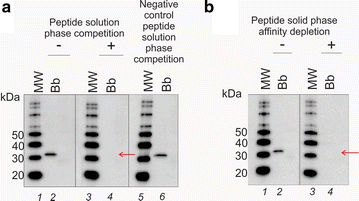
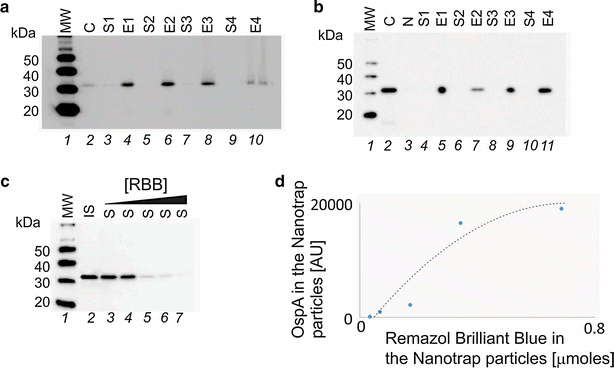
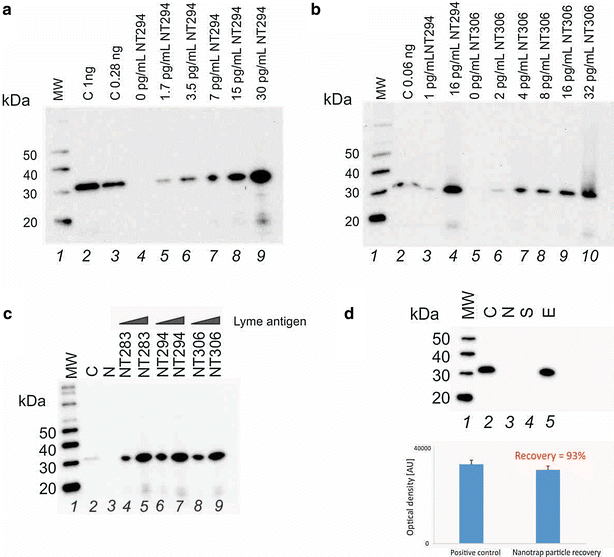
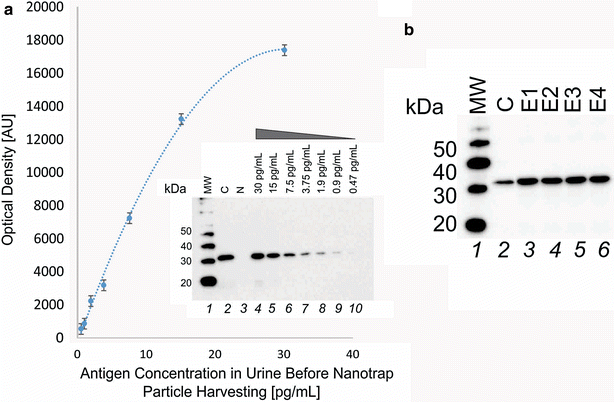
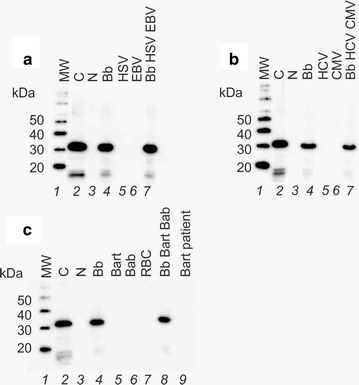
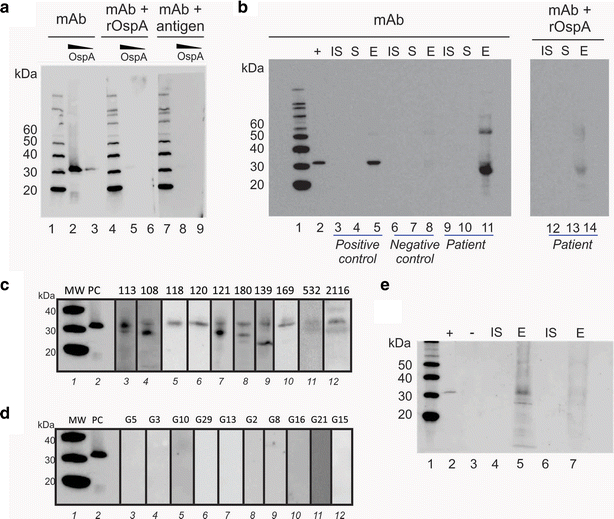
Method: We employed Nanotrap particles to concentrate urinary OspA and used a highly specific anti-OspA monoclonal antibody (mAb) as a detector of the C-terminus peptides. We mapped the mAb epitope to a narrow specific OspA C-terminal domain OspA236-239 conserved across infectious Borrelia species but with no homology to human proteins and no cross-reactivity with relevant viral and non-Borrelia bacterial proteins. 268 urine samples from patients being evaluated for all categories of LB were collected in a LB endemic area. The urinary OspA assay, blinded to outcome, utilized Nanotrap particle pre-processing, western blotting to evaluate the OspA molecular size, and OspA peptide competition for confirmation.
Results: OspA test characteristics: sensitivity 1.7 pg/mL (lowest limit of detection), % coefficient of variation (CV) = 8 %, dynamic range 1.7-30 pg/mL. Pre-treatment, 24/24 newly diagnosed patients with an erythema migrans (EM) rash were positive for urinary OspA while false positives for asymptomatic patients were 0/117 (Chi squared p < 10(-6)). For 10 patients who exhibited persistence of the EM rash during the course of antibiotic therapy, 10/10 were positive for urinary OspA. Urinary OspA of 8/8 patients switched from detectable to undetectable following symptom resolution post-treatment. Specificity of the urinary OspA test for the clinical symptoms was 40/40. Specificity of the urinary OspA antigen test for later serology outcome was 87.5 % (21 urinary OspA positive/24 serology positive, Chi squared p = 4.072e(-15)). 41 of 100 patients under surveillance for persistent LB in an endemic area were positive for urinary OspA protein.
Conclusions: OspA urinary shedding was strongly linked to concurrent active symptoms (e.g. EM rash and arthritis), while resolution of these symptoms after therapy correlated with urinary conversion to OspA negative.
Figures



References
-
- Bacon R, Kugeler K, Mead P. Centers for Disease Control and Prevention (CDC). Surveillance for Lyme disease—United States, 1992–2006. Morb Mortal Wkly Rep (MMWR) 2008; 57:SS 1-10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

