CRISPR/gRNA-directed synergistic activation mediator (SAM) induces specific, persistent and robust reactivation of the HIV-1 latent reservoirs
- PMID: 26538064
- PMCID: PMC4633726
- DOI: 10.1038/srep16277
CRISPR/gRNA-directed synergistic activation mediator (SAM) induces specific, persistent and robust reactivation of the HIV-1 latent reservoirs
Abstract
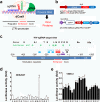
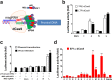
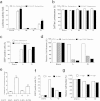
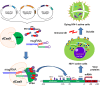
Current antiretroviral therapy does not eliminate the integrated and transcriptionally silent HIV-1 provirus in latently infected cells. Recently, a "shock and kill" strategy has been extensively explored to eradicate the HIV-1 latent reservoirs for a permanent cure of AIDS. The therapeutic efficacy of currently used agents remains disappointing because of low efficiency, non-specificity and cellular toxicity. Here we present a novel catalytically-deficient Cas9-synergistic activation mediator (dCas9-SAM) technology to selectively, potently and persistently reactivate the HIV-1 latent reservoirs. By screening 16 MS2-mediated single guide RNAs, we identified long terminal repeat (LTR)-L and O that surround the enhancer region (-165/-145 for L and -92/-112 for O) and induce robust reactivation of HIV-1 provirus in HIV-1 latent TZM-bI epithelial, Jurkat T lymphocytic and CHME5 microglial cells. This compulsory reactivation induced cellular suicide via toxic buildup of viral proteins within HIV-1 latent Jurkat T and CHME5 microglial cells. These results suggest that this highly effective and target-specific dCas9-SAM system can serve as a novel HIV-latency-reversing therapeutic tool for the permanent elimination of HIV-1 latent reservoirs.
Conflict of interest statement
A patent application has been filed relating to this work.
Figures



Comment in
-
Recent advances in CRISPR/Cas9 technology for targeting latent HIV-1 reservoirs.AIDS. 2016 Jul 17;30(11):N17. doi: 10.1097/QAD.0000000000001138. AIDS. 2016. PMID: 27139312 No abstract available.
References
-
- Sgarbanti M. & Battistini A. Therapeutics for HIV-1 reactivation from latency. Current opinion in virology 3, 394–401 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

