The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure
- PMID: 26538090
- PMCID: PMC5243220
- DOI: 10.1152/ajpcell.00248.2015
The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure
Abstract
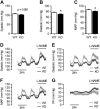
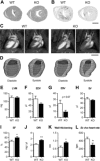
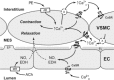
The extracellular calcium-sensing receptor CaSR is expressed in blood vessels where its role is not completely understood. In this study, we tested the hypothesis that the CaSR expressed in vascular smooth muscle cells (VSMC) is directly involved in regulation of blood pressure and blood vessel tone. Mice with targeted CaSR gene ablation from vascular smooth muscle cells (VSMC) were generated by breeding exon 7 LoxP-CaSR mice with animals in which Cre recombinase is driven by a SM22α promoter (SM22α-Cre). Wire myography performed on Cre-negative [wild-type (WT)] and Cre-positive (SM22α)CaSR(Δflox/Δflox) [knockout (KO)] mice showed an endothelium-independent reduction in aorta and mesenteric artery contractility of KO compared with WT mice in response to KCl and to phenylephrine. Increasing extracellular calcium ion (Ca(2+)) concentrations (1-5 mM) evoked contraction in WT but only relaxation in KO aortas. Accordingly, diastolic and mean arterial blood pressures of KO animals were significantly reduced compared with WT, as measured by both tail cuff and radiotelemetry. This hypotension was mostly pronounced during the animals' active phase and was not rescued by either nitric oxide-synthase inhibition with nitro-l-arginine methyl ester or by a high-salt-supplemented diet. KO animals also exhibited cardiac remodeling, bradycardia, and reduced spontaneous activity in isolated hearts and cardiomyocyte-like cells. Our findings demonstrate a role for CaSR in the cardiovascular system and suggest that physiologically relevant changes in extracellular Ca(2+) concentrations could contribute to setting blood vessel tone levels and heart rate by directly acting on the cardiovascular CaSR.
Keywords: CaSR; G protein-coupled receptor; blood pressure regulation; blood vessel tone regulation; calcium-sensing receptor; vascular smooth muscle cells.
Figures




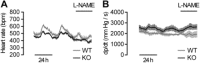
References
-
- Alam MU, Kirton JP, Wilkinson FL, Towers E, Sinha S, Rouhi M, Vizard TN, Sage AP, Martin D, Ward DT, Alexander MY, Riccardi D, Canfield AE. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res 81: 260–268, 2009. - PubMed
-
- Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drueke TB, Goodman WG. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004. - PubMed
-
- Boedtkjer E, Praetorius J, Matchkov VV, Stankevicius E, Mogensen S, Fuchtbauer AC, Simonsen U, Fuchtbauer EM, Aalkjaer C. Disruption of Na+,HCO3− cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in mice. Circulation 124: 1819–1829, 2011. - PubMed
-
- Broegger T, Jacobsen JC, Secher Dam V, Boedtkjer DM, Kold-Petersen H, Pedersen FS, Aalkjaer C, Matchkov VV. Bestrophin is important for the rhythmic but not the tonic contraction in rat mesenteric small arteries. Cardiovasc Res 91: 685–693, 2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

