Introducing an algal carbon-concentrating mechanism into higher plants: location and incorporation of key components
- PMID: 26538195
- PMCID: PMC5102585
- DOI: 10.1111/pbi.12497
Introducing an algal carbon-concentrating mechanism into higher plants: location and incorporation of key components
Abstract
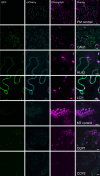
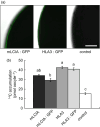
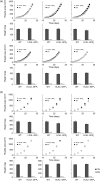
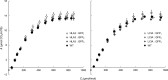
Many eukaryotic green algae possess biophysical carbon-concentrating mechanisms (CCMs) that enhance photosynthetic efficiency and thus permit high growth rates at low CO2 concentrations. They are thus an attractive option for improving productivity in higher plants. In this study, the intracellular locations of ten CCM components in the unicellular green alga Chlamydomonas reinhardtii were confirmed. When expressed in tobacco, all of these components except chloroplastic carbonic anhydrases CAH3 and CAH6 had the same intracellular locations as in Chlamydomonas. CAH6 could be directed to the chloroplast by fusion to an Arabidopsis chloroplast transit peptide. Similarly, the putative inorganic carbon (Ci) transporter LCI1 was directed to the chloroplast from its native location on the plasma membrane. CCP1 and CCP2 proteins, putative Ci transporters previously reported to be in the chloroplast envelope, localized to mitochondria in both Chlamydomonas and tobacco, suggesting that the algal CCM model requires expansion to include a role for mitochondria. For the Ci transporters LCIA and HLA3, membrane location and Ci transport capacity were confirmed by heterologous expression and H(14) CO3 (-) uptake assays in Xenopus oocytes. Both were expressed in Arabidopsis resulting in growth comparable with that of wild-type plants. We conclude that CCM components from Chlamydomonas can be expressed both transiently (in tobacco) and stably (in Arabidopsis) and retargeted to appropriate locations in higher plant cells. As expression of individual Ci transporters did not enhance Arabidopsis growth, stacking of further CCM components will probably be required to achieve a significant increase in photosynthetic efficiency in this species.
Keywords: Arabidopsis thaliana; Chlamydomonas reinhardtii; bicarbonate transporter; carbon-concentrating mechanism; photosynthesis improvement; tobacco.
© 2015 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures



References
-
- Badger, M.R. , Andrews, T.J. , Whitney, S.M. , Ludwig, M. , Yellowlees, D.C. , Leggat, W. and Price, G.D. (1998) The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast‐based CO2‐concentrating mechanisms in algae. Can. J. Bot. 76, 1052–1071.
-
- Clough, S.J. and Bent, A.F. (1998) Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . Plant J. 16, 735–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/I024453/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004561/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M006468/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M007693/1/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

