Altered morphological dynamics of activated microglia after induction of status epilepticus
- PMID: 26538404
- PMCID: PMC4634193
- DOI: 10.1186/s12974-015-0421-6
Altered morphological dynamics of activated microglia after induction of status epilepticus
Abstract
Background: Microglia cells are the resident macrophages of the central nervous system and are considered its first line of defense. In the normal brain, their ramified processes are highly motile, constantly scanning the surrounding brain tissue and rapidly moving towards sites of acute injury or danger signals. These microglial dynamics are thought to be critical for brain homeostasis. Under pathological conditions, microglial cells undergo "activation," which modifies many of their molecular and morphological properties. Investigations of the effects of activation on motility are limited and have given mixed results. In particular, little is known about how microglial motility is altered in epilepsy, which is characterized by a strong inflammatory reaction and microglial activation.
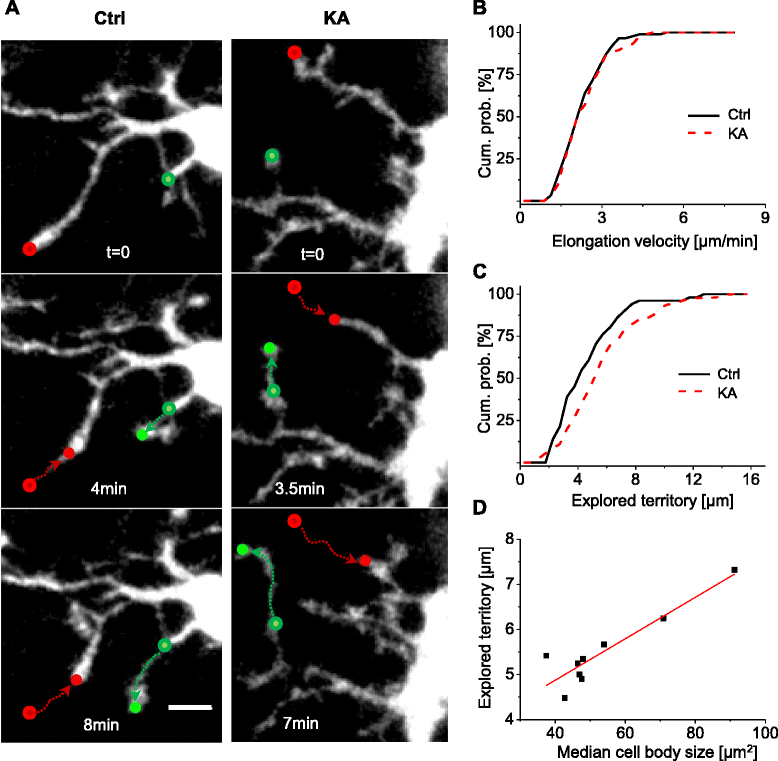
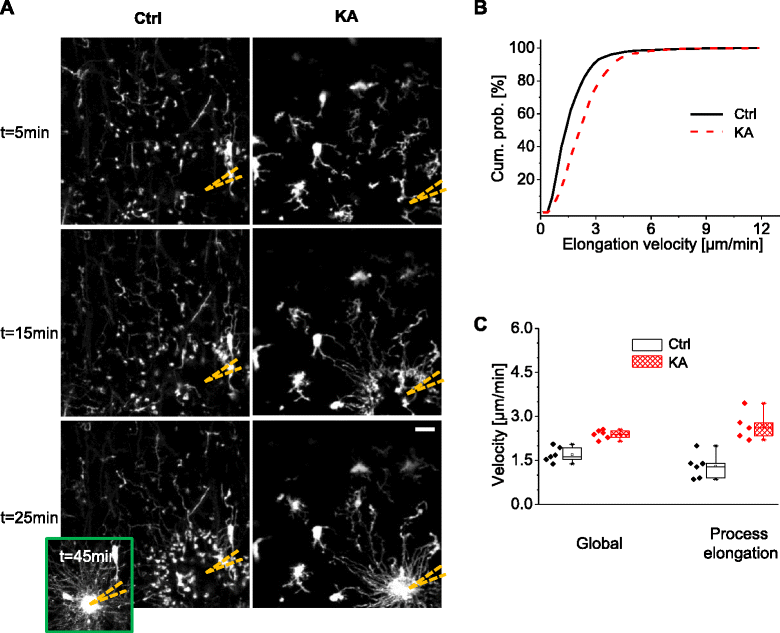
Methods: We used a mouse model of status epilepticus induced by kainate injections and time-lapse two-photon microscopy to image GFP-labeled microglia in acute hippocampal brain slices. We studied how microglial activation affected the motility of microglial processes, including basal motility, and their responses to local triggering stimuli.
Results: Our study reveals that microglial motility was largely preserved in kainate-treated animals, despite clear signs of microglial activation. In addition, whereas the velocities of microglial processes during basal scanning and towards a laser lesion were unaltered 48 h after status epilepticus, we observed an increase in the size of the territory scanned by single microglial processes during basal motility and an elevated directional velocity towards a pipette containing a purinergic agonist.
Conclusions: Microglial activation differentially impacted the dynamic scanning behavior of microglia in response to specific acute noxious stimuli, which may be an important feature of the adaptive behavior of microglia during pathophysiological conditions.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

