Distinct Cell- and Layer-Specific Expression Patterns and Independent Regulation of Kv2 Channel Subtypes in Cortical Pyramidal Neurons
- PMID: 26538660
- PMCID: PMC4635138
- DOI: 10.1523/JNEUROSCI.1897-15.2015
Distinct Cell- and Layer-Specific Expression Patterns and Independent Regulation of Kv2 Channel Subtypes in Cortical Pyramidal Neurons
Abstract
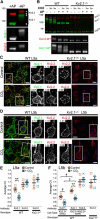
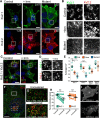
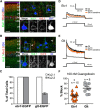
The Kv2 family of voltage-gated potassium channel α subunits, comprising Kv2.1 and Kv2.2, mediate the bulk of the neuronal delayed rectifier K(+) current in many mammalian central neurons. Kv2.1 exhibits robust expression across many neuron types and is unique in its conditional role in modulating intrinsic excitability through changes in its phosphorylation state, which affect Kv2.1 expression, localization, and function. Much less is known of the highly related Kv2.2 subunit, especially in forebrain neurons. Here, through combined use of cortical layer markers and transgenic mouse lines, we show that Kv2.1 and Kv2.2 are localized to functionally distinct cortical cell types. Kv2.1 expression is consistently high throughout all cortical layers, especially in layer (L) 5b pyramidal neurons, whereas Kv2.2 expression is primarily limited to neurons in L2 and L5a. In addition, L4 of primary somatosensory cortex is strikingly devoid of Kv2.2 immunolabeling. The restricted pattern of Kv2.2 expression persists in Kv2.1-KO mice, suggesting distinct cell- and layer-specific functions for these two highly related Kv2 subunits. Analyses of endogenous Kv2.2 in cortical neurons in situ and recombinant Kv2.2 expressed in heterologous cells reveal that Kv2.2 is largely refractory to stimuli that trigger robust, phosphorylation-dependent changes in Kv2.1 clustering and function. Immunocytochemistry and voltage-clamp recordings from outside-out macropatches reveal distinct cellular expression patterns for Kv2.1 and Kv2.2 in intratelencephalic and pyramidal tract neurons of L5, indicating circuit-specific requirements for these Kv2 paralogs. Together, these results support distinct roles for these two Kv2 channel family members in mammalian cortex.
Significance statement: Neurons within the neocortex are arranged in a laminar architecture and contribute to the input, processing, and/or output of sensory and motor signals in a cell- and layer-specific manner. Neurons of different cortical layers express diverse populations of ion channels and possess distinct intrinsic membrane properties. Here, we show that the Kv2 family members Kv2.1 and Kv2.2 are expressed in distinct cortical layers and pyramidal cell types associated with specific corticostriatal pathways. We find that Kv2.1 and Kv2.2 exhibit distinct responses to acute phosphorylation-dependent regulation in brain neurons in situ and in heterologous cells in vitro. These results identify a molecular mechanism that contributes to heterogeneity in cortical neuron ion channel function and regulation.
Keywords: electrophysiology; immunohistochemistry; localization; modulation; neocortex; network.
Copyright © 2015 the authors 0270-6474/15/3514923-21$15.00/0.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
