Evaluation of the ability of natural and synthetic scaffolds in providing an appropriate environment for growth and chondrogenic differentiation of adipose-derived mesenchymal stem cells
- PMID: 26538764
- PMCID: PMC4598549
- DOI: 10.4103/0019-5413.164043
Evaluation of the ability of natural and synthetic scaffolds in providing an appropriate environment for growth and chondrogenic differentiation of adipose-derived mesenchymal stem cells
Retraction in
-
Retraction Note to: Evaluation of the Ability of Natural and Synthetic Scaffolds in Providing an Appropriate Environment for Growth and Chondrogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells.Indian J Orthop. 2022 Sep 9;56(10):1841. doi: 10.1007/s43465-022-00738-w. eCollection 2022 Oct. Indian J Orthop. 2022. PMID: 36187583 Free PMC article.
Abstract
Background: Although progenitor cells have been observed in articular cartilage, this part has a limited ability to repair due to a lack of blood supply. Formerly, tissue engineering was mainly based on collecting chondrocytes from the joint surface, culturing them on resorbable scaffolds such as poly D, L-lactic glycolic acid (PLGA) and then autologous transplantation. In recent times, due to difficulties in collecting chondrocytes, most of the researchers are focused on stem cells for producing these cells. Among the important factors in this approach, is using appropriate scaffolds with good mechanical and biological properties to provide optimal environment for growth and development of stem cells. In this study, we evaluated the potential of fibrin glue, PLGA and alginate scaffolds in providing a suitable environment for growth and chondrogenic differentiation of mesenchymal stem cells (MSCs) in the presence of transforming growth factor-β3.
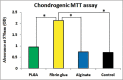
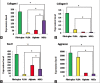
Materials and methods: Fibrin glue, PLGA and alginate scaffolds were prepared and MSCs were isolated from human adipose tissue. Cells were cultured separately on the scaffolds and 2 weeks after differentiation, chondrogenic genes, cell proliferation ability and morphology in each scaffold were evaluated using real time-polymerase chain reaction, MTT chondrogenic assay and histological examination, respectively.
Results: Proliferation of differentiated adipose tissue derived mesenchymal stem cells (AD-MSCs) to chondrogenic cells in Fibrin glue were significantly higher than in other scaffolds. Also, Fibrin glue caused the highest expression of chondrogenic genes compared to the other scaffolds. Histological examination revealed that the pores of the Fibrin glue scaffolds were filled with cells uniformly distributed.
Conclusion: According to the results of the study, it can be concluded that natural scaffolds such as fibrin can be used as an appropriate environment for cartilage differentiation.
Keywords: Adipose-derived mesenchymal stem cells; L-lactic glycolic acid; Stem cells; alginate; fibrin glue; growth factors; poly D; stem cell research; tissue engineering.
Conflict of interest statement
Figures


References
-
- Khan IM, Gilbert SJ, Singhrao SK, Duance VC, Archer CW. Cartilage integration: Evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater. 2008;16:26–39. - PubMed
-
- Yarlagadda PK, Chandrasekharan M, Shyan JY. Recent advances and current developments in tissue scaffolding. Biomed Mater Eng. 2005;15:159–77. - PubMed
-
- Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: A review. Int J Polym Sci. 2011;290602:1–19.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
