Characteristics of structures and lesions of the eye in laboratory animals used in toxicity studies
- PMID: 26538807
- PMCID: PMC4604127
- DOI: 10.1293/tox.2015-0037
Characteristics of structures and lesions of the eye in laboratory animals used in toxicity studies
Erratum in
-
Errata (Printer's correction).J Toxicol Pathol. 2016 Jan;29(1):74. Epub 2016 Feb 17. J Toxicol Pathol. 2016. PMID: 26989306 Free PMC article.
Abstract
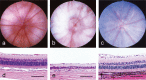
Histopathology of the eye is an essential part of ocular toxicity evaluation. There are structural variations of the eye among several laboratory animals commonly used in toxicity studies, and many cases of ocular lesions in these animals are related to anatomical and physiological characteristics of the eye. Since albino rats have no melanin in the eye, findings of the fundus can be observed clearly by ophthalmoscopy. Retinal atrophy is observed as a hyper-reflective lesion in the fundus and is usually observed as degeneration of the retina in histopathology. Albino rats are sensitive to light, and light-induced retinal degeneration is commonly observed because there is no melanin in the eye. Therefore, it is important to differentiate the causes of retinal degeneration because the lesion occurs spontaneously and is induced by several drugs or by lighting. In dogs, the tapetum lucidum, a multilayered reflective tissue of the choroid, is one of unique structures of the eye. Since tapetal cells contain reflecting crystals in which a high level of zinc has been demonstrated chemically, drug-induced tapetum degeneration is possibly related to zinc chelation. The eye of the monkey has a macula similar to that of humans. The macula consists only of cones with a high density, and light falls directly on the macula that plays an important role in visual acuity. Macular degeneration occurring in monkeys resembles histopathologically that of humans. Hence, the eye of the monkey is a suitable model to investigate macular degeneration and to assess drug-induced macular lesions.
Keywords: eye; laboratory animals; lesions; structures.
Figures


References
-
- OECD. Test Guideline 408. Repeated dose 90-day oral toxicity study in rodents. 1998.
-
- OECD. Test Guideline 409. Repeated dose 90-day oral toxicity study in non-rodents. 1998.
-
- OECD. Test Guideline 452. Chronic toxicity studies. 2009.
-
- OECD. Test Guideline 453. Combined chronic toxicity/carcinogenicity studies. 2009.
-
- Onodera H, Sasaki S, Otake S, Tomohiro M, Shibuya K, and Nomura M. General considerations in ocular toxicity risk assessment from the toxicologists’ viewpoints. J Toxicol Sci. 40: 295–307. 2015. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
