Marine Diterpenoids as Potential Anti-Inflammatory Agents
- PMID: 26538822
- PMCID: PMC4619941
- DOI: 10.1155/2015/263543
Marine Diterpenoids as Potential Anti-Inflammatory Agents
Abstract
The inflammatory response is a highly regulated process, and its dysregulation can lead to the establishment of chronic inflammation and, in some cases, to death. Inflammation is the cause of several diseases, including rheumatoid arthritis, inflammatory bowel diseases, multiple sclerosis, and asthma. The search for agents inhibiting inflammation is a great challenge as the inflammatory response plays an important role in the defense of the host to infections. Marine invertebrates are exceptional sources of new natural products, and among those diterpenoids secondary metabolites exhibit notable anti-inflammatory properties. Novel anti-inflammatory diterpenoids, exclusively produced by marine organisms, have been identified and synthetic molecules based on those structures have been obtained. The anti-inflammatory activity of marine diterpenoids has been attributed to the inhibition of Nuclear Factor-κB activation and to the modulation of arachidonic acid metabolism. However, more research is necessary to describe the mechanisms of action of these secondary metabolites. This review is a compilation of marine diterpenoids, mainly isolated from corals, which have been described as potential anti-inflammatory molecules.
Figures

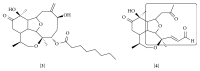
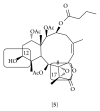
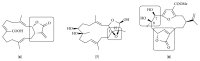
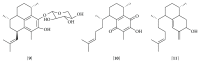
References
-
- Libby P. Inflammation and cardiovascular disease mechanisms. The American Journal of Clinical Nutrition. 2006;83(2):456S–460S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

