Friends or Foes: Matrix Metalloproteinases and Their Multifaceted Roles in Neurodegenerative Diseases
- PMID: 26538832
- PMCID: PMC4619970
- DOI: 10.1155/2015/620581
Friends or Foes: Matrix Metalloproteinases and Their Multifaceted Roles in Neurodegenerative Diseases
Abstract
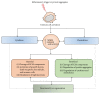
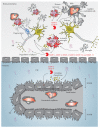
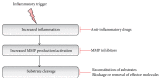
Neurodegeneration is a chronic progressive loss of neuronal cells leading to deterioration of central nervous system (CNS) functionality. It has been shown that neuroinflammation precedes neurodegeneration in various neurodegenerative diseases. Matrix metalloproteinases (MMPs), a protein family of zinc-containing endopeptidases, are essential in (neuro)inflammation and might be involved in neurodegeneration. Although MMPs are indispensable for physiological development and functioning of the organism, they are often referred to as double-edged swords due to their ability to also inflict substantial damage in various pathological conditions. MMP activity is strictly controlled, and its dysregulation leads to a variety of pathologies. Investigation of their potential use as therapeutic targets requires a better understanding of their contributions to the development of neurodegenerative diseases. Here, we review MMPs and their roles in neurodegenerative diseases: Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), Huntington's disease (HD), and multiple sclerosis (MS). We also discuss MMP inhibition as a possible therapeutic strategy to treat neurodegenerative diseases.
Figures

References
-
- Gorkiewicz T., Balcerzyk M., Kaczmarek L., Knapska E. Matrix metalloproteinase 9 (MMP-9) is indispensable for long term potentiation in the central and basal but not in the lateral nucleus of the amygdala. Frontiers in Cellular Neuroscience. 2015;9, article 73 doi: 10.3389/fncel.2015.00073. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

