Extracellular vesicles from infected cells: potential for direct pathogenesis
- PMID: 26539170
- PMCID: PMC4611157
- DOI: 10.3389/fmicb.2015.01132
Extracellular vesicles from infected cells: potential for direct pathogenesis
Abstract
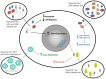
Infections that result in natural or manmade spread of lethal biological agents are a concern and require national and focused preparedness. In this manuscript, as part of an early diagnostics and pathogen treatment strategy, we have focused on extracellular vesicles (EVs) that arise following infections. Although the field of biodefense does not currently have a rich resource in EVs literature, none the less, similar pathogens belonging to the more classical emerging and non-emerging diseases have been studied in their EV/exosomal contents and function. These exosomes are formed in late endosomes and released from the cell membrane in almost every cell type in vivo. These vesicles contain proteins, RNA, and lipids from the cells they originate from and function in development, signal transduction, cell survival, and transfer of infectious material. The current review focuses on how different forms of infection exploit the exosomal pathway and how exosomes can be exploited artificially to treat infection and disease and potentially also be used as a source of vaccine. Virally-infected cells can secrete viral as well as cellular proteins and RNA in exosomes, allowing viruses to cause latent infection and spread of miRNA to nearby cells prior to a subsequent infection. In addition to virally-infected host cells, bacteria, protozoa, and fungi can all release small vesicles that contain pathogen-associated molecular patterns, regulating the neighboring uninfected cells. Examples of exosomes from both virally and bacterially infected cells point toward a re-programming network of pathways in the recipient cells. Finally, many of these exosomes contain cytokines and miRNAs that in turn can effect gene expression in the recipient cells through the classical toll-like receptor and NFκB pathway. Therefore, although exosomes do not replicate as an independent entity, they however facilitate movement of infectious material through tissues and may be the cause of many pathologies seen in infected hosts.
Keywords: bacteria; exosome; extracellular vesicle; parasite; pathogen; protozoa; virus.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

