A loopy view of telomere evolution
- PMID: 26539211
- PMCID: PMC4612135
- DOI: 10.3389/fgene.2015.00321
A loopy view of telomere evolution
Abstract
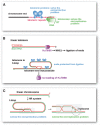
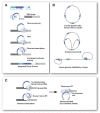
About a decade ago, I proposed that t-loops, the lariat structures adopted by many eukaryotic telomeres, could explain how the transition from circular to linear chromosomes was successfully negotiated by early eukaryotes. Here I reconsider this loopy hypothesis in the context of the idea that eukaryotes evolved through a period of genome invasion by Group II introns.
Keywords: DNA damage; Group II intron; eukaryote; replication; telomerase; telomere.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

