Soluble Urokinase Receptor and Chronic Kidney Disease
- PMID: 26539835
- PMCID: PMC4701036
- DOI: 10.1056/NEJMoa1506362
Soluble Urokinase Receptor and Chronic Kidney Disease
Abstract
Background: Relatively high plasma levels of soluble urokinase-type plasminogen activator receptor (suPAR) have been associated with focal segmental glomerulosclerosis and poor clinical outcomes in patients with various conditions. It is unknown whether elevated suPAR levels in patients with normal kidney function are associated with future decline in the estimated glomerular filtration rate (eGFR) and with incident chronic kidney disease.
Methods: We measured plasma suPAR levels in 3683 persons enrolled in the Emory Cardiovascular Biobank (mean age, 63 years; 65% men; median suPAR level, 3040 pg per milliliter) and determined renal function at enrollment and at subsequent visits in 2292 persons. The relationship between suPAR levels and the eGFR at baseline, the change in the eGFR over time, and the development of chronic kidney disease (eGFR <60 ml per minute per 1.73 m(2) of body-surface area) were analyzed with the use of linear mixed models and Cox regression after adjustment for demographic and clinical variables.
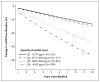
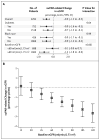
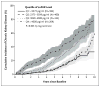
Results: A higher suPAR level at baseline was associated with a greater decline in the eGFR during follow-up; the annual change in the eGFR was -0.9 ml per minute per 1.73 m(2) among participants in the lowest quartile of suPAR levels as compared with -4.2 ml per minute per 1.73 m(2) among participants in the highest quartile (P<0.001). The 921 participants with a normal eGFR (≥ 90 ml per minute per 1.73 m(2)) at baseline had the largest suPAR-related decline in the eGFR. In 1335 participants with a baseline eGFR of at least 60 ml per minute per 1.73 m(2), the risk of progression to chronic kidney disease in the highest quartile of suPAR levels was 3.13 times as high (95% confidence interval, 2.11 to 4.65) as that in the lowest quartile.
Conclusions: An elevated level of suPAR was independently associated with incident chronic kidney disease and an accelerated decline in the eGFR in the groups studied. (Funded by the Abraham J. and Phyllis Katz Foundation and others.).
Figures
Comment in
-
A suPAR Biomarker for Chronic Kidney Disease.N Engl J Med. 2015 Nov 12;373(20):1971-2. doi: 10.1056/NEJMe1512997. Epub 2015 Nov 5. N Engl J Med. 2015. PMID: 26539740 No abstract available.
-
New blood marker can detect chronic kidney disease, study shows.BMJ. 2015 Nov 8;351:h5894. doi: 10.1136/bmj.h5894. BMJ. 2015. PMID: 26552837 No abstract available.
-
Chronic kidney disease: suPAR in CKD.Nat Rev Nephrol. 2016 Jan;12(1):3. doi: 10.1038/nrneph.2015.195. Epub 2015 Nov 23. Nat Rev Nephrol. 2016. PMID: 26592192 No abstract available.
-
Soluble Urokinase Receptor and Chronic Kidney Disease.N Engl J Med. 2016 Mar 3;374(9):891. doi: 10.1056/NEJMc1515787. N Engl J Med. 2016. PMID: 26962914 No abstract available.
-
Soluble Urokinase Receptor and Chronic Kidney Disease.N Engl J Med. 2016 Mar 3;374(9):890. doi: 10.1056/NEJMc1515787. N Engl J Med. 2016. PMID: 26962915 No abstract available.
-
Soluble Urokinase Receptor and Chronic Kidney Disease.N Engl J Med. 2016 Mar 3;374(9):890. doi: 10.1056/NEJMc1515787. N Engl J Med. 2016. PMID: 26962916 No abstract available.
References
-
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. - PubMed
-
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–52. - PubMed
-
- James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375:1296–309. - PubMed
-
- Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL089650/HL/NHLBI NIH HHS/United States
- U01 AI103397/AI/NIAID NIH HHS/United States
- U01-AI-103390/AI/NIAID NIH HHS/United States
- R01 DK101350/DK/NIDDK NIH HHS/United States
- U01-AI-103408/AI/NIAID NIH HHS/United States
- U01-AI-42590/AI/NIAID NIH HHS/United States
- U01 AI031834/AI/NIAID NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01-AI-103401/AI/NIAID NIH HHS/United States
- R01 HL113451/HL/NHLBI NIH HHS/United States
- U01-AI-34994/AI/NIAID NIH HHS/United States
- U01-AI-35004/AI/NIAID NIH HHS/United States
- U01-AI-31834/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U01-AI-103397/AI/NIAID NIH HHS/United States
- U01 AI103401/AI/NIAID NIH HHS/United States
- U01 AI103390/AI/NIAID NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- P20 HL113451/HL/NHLBI NIH HHS/United States
- U01 AI103408/AI/NIAID NIH HHS/United States
- U01-AI-34989/AI/NIAID NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
