L-Stepholidine rescues memory deficit and synaptic plasticity in models of Alzheimer's disease via activating dopamine D1 receptor/PKA signaling pathway
- PMID: 26539912
- PMCID: PMC4670924
- DOI: 10.1038/cddis.2015.315
L-Stepholidine rescues memory deficit and synaptic plasticity in models of Alzheimer's disease via activating dopamine D1 receptor/PKA signaling pathway
Abstract
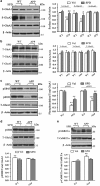
It is accepted that amyloid β-derived diffusible ligands (ADDLs) have a prominent role in triggering the early cognitive deficits that constitute Alzheimer's disease (AD). However, there is still no effective treatment for preventing or reversing the progression of the disease. Targeting α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor trafficking and its regulation is a new strategy for AD early treatment. Here we investigate the effect and mechanism of L-Stepholidine (L-SPD), which elicits dopamine D1-type receptor agonistic activity, while acting as D2-type receptor antagonist on cognition and synaptic plasticity in amyloid precursor protein (APP) and presenilin 1 (PS1) double-transgenic (APP/PS1) mice, and hippocampal cultures or slices treated with ADDLs. L-SPD could improve the hippocampus-dependent memory, surface expression of glutamate receptor A (GluA1)-containing AMPA receptors and spine density in hippocampus of APP/PS1 transgenic mice. L-SPD not only rescued decreased phosphorylation and surface expression of GluA1 in hippocampal cultures but also protected the long-term potentiation in hippocampal slices induced by ADDLs. Protein kinase A (PKA) agonist Sp-cAMPS or D1-type receptor agonist SKF81297 had similar effects, whereas PKA antagonist Rp-cAMPS or D1-type receptor antagonist SCH23390 abolished the effect of L-SPD on GluA1 trafficking. This was mediated mainly by PKA, which could phosphorylate serine residue at 845 of the GluA1. L-SPD may be explored as a potential therapeutic drug for AD through a mechanism that improves AMPA receptor trafficking and synaptic plasticity via activating D1/PKA signaling pathway.
Figures






Similar articles
-
Synaptic modification by L-theanine, a natural constituent in green tea, rescues the impairment of hippocampal long-term potentiation and memory in AD mice.Neuropharmacology. 2018 Aug;138:331-340. doi: 10.1016/j.neuropharm.2018.06.030. Epub 2018 Jun 23. Neuropharmacology. 2018. PMID: 29944861
-
l-Stepholidine increases the frequency of sEPSC via the activation of D1 dopamine signaling pathway in rat prelimbic cortical neurons.Acta Pharmacol Sin. 2007 May;28(5):627-33. doi: 10.1111/j.1745-7254.2007.00547.x. Acta Pharmacol Sin. 2007. PMID: 17439718
-
Blocking the Interaction between EphB2 and ADDLs by a Small Peptide Rescues Impaired Synaptic Plasticity and Memory Deficits in a Mouse Model of Alzheimer's Disease.J Neurosci. 2016 Nov 23;36(47):11959-11973. doi: 10.1523/JNEUROSCI.1327-16.2016. J Neurosci. 2016. PMID: 27881781 Free PMC article.
-
(-)-Stepholidine: a potential novel antipsychotic drug with dual D1 receptor agonist and D2 receptor antagonist actions.Trends Pharmacol Sci. 2002 Jan;23(1):4-7. doi: 10.1016/s0165-6147(00)01929-5. Trends Pharmacol Sci. 2002. PMID: 11804640 Review. No abstract available.
-
[Preventive action of nobiletin, a constituent of AURANTII NOBILIS PERICARPIUM with anti-dementia activity, against amyloid-beta peptide-induced neurotoxicity expression and memory impairment].Yakugaku Zasshi. 2010 Apr;130(4):517-20. doi: 10.1248/yakushi.130.517. Yakugaku Zasshi. 2010. PMID: 20371995 Review. Japanese.
Cited by
-
Overexpression of EphB2 in hippocampus rescues impaired NMDA receptors trafficking and cognitive dysfunction in Alzheimer model.Cell Death Dis. 2017 Mar 30;8(3):e2717. doi: 10.1038/cddis.2017.140. Cell Death Dis. 2017. PMID: 28358367 Free PMC article.
-
Understanding How Physical Exercise Improves Alzheimer's Disease: Cholinergic and Monoaminergic Systems.Front Aging Neurosci. 2022 May 18;14:869507. doi: 10.3389/fnagi.2022.869507. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35663578 Free PMC article. Review.
-
Synthesis and Inhibition Evaluation of New Benzyltetrahydroprotoberberine Alkaloids Designed as Acetylcholinesterase Inhibitors.Front Chem. 2019 Sep 18;7:629. doi: 10.3389/fchem.2019.00629. eCollection 2019. Front Chem. 2019. PMID: 31620424 Free PMC article.
-
Tryptophan-Tyrosine Dipeptide, the Core Sequence of β-Lactolin, Improves Memory by Modulating the Dopamine System.Nutrients. 2019 Feb 6;11(2):348. doi: 10.3390/nu11020348. Nutrients. 2019. PMID: 30736353 Free PMC article.
-
A critical review: traditional uses, phytochemistry, pharmacology and toxicology of Stephania tetrandra S. Moore (Fen Fang Ji).Phytochem Rev. 2020;19(2):449-489. doi: 10.1007/s11101-020-09673-w. Epub 2020 Apr 24. Phytochem Rev. 2020. PMID: 32336965 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

