Bimanual motor coordination controlled by cooperative interactions in intrinsic and extrinsic coordinates
- PMID: 26540267
- PMCID: PMC4738419
- DOI: 10.1111/ejn.13123
Bimanual motor coordination controlled by cooperative interactions in intrinsic and extrinsic coordinates
Abstract
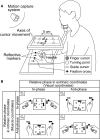
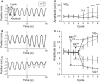
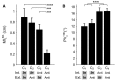
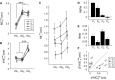
Although strong motor coordination in intrinsic muscle coordinates has frequently been reported for bimanual movements, coordination in extrinsic visual coordinates is also crucial in various bimanual tasks. To explore the bimanual coordination mechanisms in terms of the frame of reference, here we characterized implicit bilateral interactions in visuomotor tasks. Visual perturbations (finger-cursor gain change) were applied while participants performed a rhythmic tracking task with both index fingers under an in-phase or anti-phase relationship in extrinsic coordinates. When they corrected the right finger's amplitude, the left finger's amplitude unintentionally also changed [motor interference (MI)], despite the instruction to keep its amplitude constant. Notably, we observed two specificities: one was large MI and low relative-phase variability (PV) under the intrinsic in-phase condition, and the other was large MI and high PV under the extrinsic in-phase condition. Additionally, using a multiple-interaction model, we successfully decomposed MI into intrinsic components caused by motor correction and extrinsic components caused by visual-cursor mismatch of the right finger's movements. This analysis revealed that the central nervous system facilitates MI by combining intrinsic and extrinsic components in the condition with in-phases in both intrinsic and extrinsic coordinates, and that under-additivity of the effects is explained by the brain's preference for the intrinsic interaction over extrinsic interaction. In contrast, the PV was significantly correlated with the intrinsic component, suggesting that the intrinsic interaction dominantly contributed to bimanual movement stabilization. The inconsistent features of MI and PV suggest that the central nervous system regulates multiple levels of bilateral interactions for various bimanual tasks.
Keywords: bilateral interaction; cyclic movement; frame of reference; human intermanual coordination; relative phase stability.
© 2015 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures

References
-
- Byblow, W.D. , Lewis, G.N. , Stinear, J.W. , Austin, N.J. & Lynch, M. (2000) The subdominant hand increases in the efficacy of voluntary alterations in bimanual coordination. Exp. Brain Res., 131, 366–374. - PubMed
-
- Carson, R.G. (1995) The dynamics of isometric bimanual coordination. Exp. Brain Res., 105, 465–476. - PubMed
-
- Carson, R.G. (2005) Neural pathways mediating bilateral interactions between the upper limbs. Brain Res. Brain Res. Rev., 49, 641–662. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

