Changes in autophagy, proteasome activity and metabolism to determine a specific signature for acute and chronic senescent mesenchymal stromal cells
- PMID: 26540573
- PMCID: PMC4741838
- DOI: 10.18632/oncotarget.6277
Changes in autophagy, proteasome activity and metabolism to determine a specific signature for acute and chronic senescent mesenchymal stromal cells
Abstract
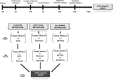
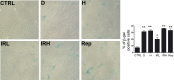
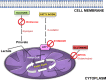
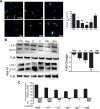
A sharp definition of what a senescent cell is still lacking since we do not have in depth understanding of mechanisms that induce cellular senescence. In addition, senescent cells are heterogeneous, in that not all of them express the same genes and present the same phenotype. To further clarify the classification of senescent cells, hints may be derived by the study of cellular metabolism, autophagy and proteasome activity. In this scenario, we decided to study these biological features in senescence of Mesenchymal Stromal Cells (MSC). These cells contain a subpopulation of stem cells that are able to differentiate in mesodermal derivatives (adipocytes, chondrocytes, osteocytes). In addition, they can also contribute to the homeostatic maintenance of many organs, hence, their senescence could be very deleterious for human body functions. We induced MSC senescence by oxidative stress, doxorubicin treatment, X-ray irradiation and replicative exhaustion. The first three are considered inducers of acute senescence while extensive proliferation triggers replicative senescence also named as chronic senescence. In all conditions, but replicative and high IR dose senescence, we detected a reduction of the autophagic flux, while proteasome activity was impaired in peroxide-treated and irradiated cells. Differences were observed also in metabolic status. In general, all senescent cells evidenced metabolic inflexibility and prefer to use glucose as energy fuel. Irradiated cells with low dose of X-ray and replicative senescent cells show a residual capacity to use fatty acids and glutamine as alternative fuels, respectively. Our study may be useful to discriminate among different senescent phenotypes.
Keywords: Gerotarget; autophagy; mesenchymal stem cells; metabolism; proteasome; senescence.
Conflict of interest statement
None of the authors have competing interests.
Figures

References
-
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. - PubMed
-
- Galderisi U, Giordano A. The gap between the physiological and therapeutic roles of mesenchymal stem cells. Medicinal research reviews. 2014;34(5):1100–1126. - PubMed
-
- Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. Journal of cellular physiology. 2007;211(1):27–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

