Integrative analysis to select cancer candidate biomarkers to targeted validation
- PMID: 26540631
- PMCID: PMC4791256
- DOI: 10.18632/oncotarget.6018
Integrative analysis to select cancer candidate biomarkers to targeted validation
Abstract
Targeted proteomics has flourished as the method of choice for prospecting for and validating potential candidate biomarkers in many diseases. However, challenges still remain due to the lack of standardized routines that can prioritize a limited number of proteins to be further validated in human samples. To help researchers identify candidate biomarkers that best characterize their samples under study, a well-designed integrative analysis pipeline, comprising MS-based discovery, feature selection methods, clustering techniques, bioinformatic analyses and targeted approaches was performed using discovery-based proteomic data from the secretomes of three classes of human cell lines (carcinoma, melanoma and non-cancerous). Three feature selection algorithms, namely, Beta-binomial, Nearest Shrunken Centroids (NSC), and Support Vector Machine-Recursive Features Elimination (SVM-RFE), indicated a panel of 137 candidate biomarkers for carcinoma and 271 for melanoma, which were differentially abundant between the tumor classes. We further tested the strength of the pipeline in selecting candidate biomarkers by immunoblotting, human tissue microarrays, label-free targeted MS and functional experiments. In conclusion, the proposed integrative analysis was able to pre-qualify and prioritize candidate biomarkers from discovery-based proteomics to targeted MS.
Keywords: candidate biomarker; discovery; integrative analysis; proteomics; targeted.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

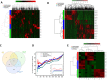
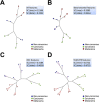



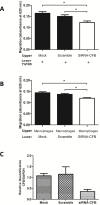
References
-
- Kulasingam V, Diamandis EP. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nature clinical practice Oncology. 2008;5:588–99. - PubMed
-
- Chen R, Pan S, Brentnall TA, Aebersold R. Proteomic profiling of pancreatic cancer for biomarker discovery. Molecular & cellular proteomics: MCP. 2005;4:523–33. - PubMed
-
- White NM, Masui O, Desouza LV, Krakovska O, Metias S, Romaschin AD, Honey RJ, Stewart R, Pace K, Lee J, Jewett MA, Bjarnason GA, Siu KW, et al. Quantitative proteomic analysis reveals potential diagnostic markers and pathways involved in pathogenesis of renal cell carcinoma. Oncotarget. 2014;5:506–18. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

