Human Adult Retinal Pigment Epithelial Stem Cell-Derived RPE Monolayers Exhibit Key Physiological Characteristics of Native Tissue
- PMID: 26540654
- PMCID: PMC4640474
- DOI: 10.1167/iovs.14-16246
Human Adult Retinal Pigment Epithelial Stem Cell-Derived RPE Monolayers Exhibit Key Physiological Characteristics of Native Tissue
Abstract
Purpose: We tested what native features have been preserved with a new culture protocol for adult human RPE.
Methods: We cultured RPE from adult human eyes. Standard protocols for immunohistochemistry, electron microscopy, electrophysiology, fluid transport, and ELISA were used.
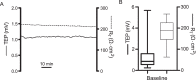
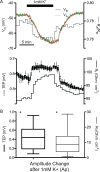
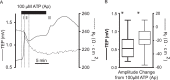
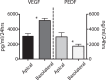
Results: Confluent monolayers of adult human RPE cultures exhibit characteristics of native RPE. Immunohistochemistry demonstrated polarized expression of RPE markers. Electron microscopy illustrated characteristics of native RPE. The mean transepithelial potential (TEP) was 1.19 ± 0.24 mV (mean ± SEM, n = 31), apical positive, and the mean transepithelial resistance (RT) was 178.7 ± 9.9 Ω·cm2 (mean ± SEM, n = 31). Application of 100 μM adenosine triphosphate (ATP) apically increased net fluid absorption (Jv) by 6.11 ± 0.53 μL·cm2·h-1 (mean ± SEM, n = 6) and TEP by 0.33 ± 0.048 mV (mean ± SEM, n = 25). Gene expression of cultured RPE was comparable to native adult RPE (n = 5); however, native RPE RNA was harvested between 24 and 40 hours after death and, therefore, may not accurately reflect healthy native RPE. Vascular endothelial growth factor secreted preferentially basally 2582 ± 146 pg/mL/d, compared to an apical secretion of 1548 ± 162 pg/mL/d (n = 14, P < 0.01), while PEDF preferentially secreted apically 1487 ± 280 ng/mL/d compared to a basolateral secretion of 864 ± 132 ng/mL/d (n = 14, P < 0.01).
Conclusions: The new culture model preserves native RPE morphology, electrophysiology, and gene and protein expression patterns, and may be a useful model to study RPE physiology, disease, and transplantation.
Figures







References
-
- Zarbin MA,, Rosenfeld PJ. Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina. 2010; 30: 1350–1367. - PubMed
-
- Kolomeyer AM,, Zarbin MA. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv Ophthalmol. 2014; 59: 134–165. - PubMed
-
- Gallemore RP,, Hughes BA,, Miller SS. Light-induced responses of the retinal pigment epithelium. : Marmor MF,, Wolfensburger TJ, Retinal Pigment Epithelium: Current Aspects of Function and Disease. Oxford: Oxford University Press; 1998: 103–134.
-
- Maminishkis A,, Jalickee S,, Blaug SA,, et al. The P2Y(2) receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Invest Ophthalmol Vis Sci. 2002; 43: 3555–3566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

