Treatment of Inherited Eye Defects by Systemic Hematopoietic Stem Cell Transplantation
- PMID: 26540660
- PMCID: PMC4640476
- DOI: 10.1167/iovs.15-17107
Treatment of Inherited Eye Defects by Systemic Hematopoietic Stem Cell Transplantation
Abstract
Purpose: Cystinosis is caused by a deficiency in the lysosomal cystine transporter, cystinosin (CTNS gene), resulting in cystine crystal accumulation in tissues. In eyes, crystals accumulate in the cornea causing photophobia and eventually blindness. Hematopoietic stem progenitor cells (HSPCs) rescue the kidney in a mouse model of cystinosis. We investigated the potential for HSPC transplantation to treat corneal defects in cystinosis.
Methods: We isolated HSPCs from transgenic DsRed mice and systemically transplanted irradiated Ctns-/- mice. A year posttransplantation, we investigated the fate and function of HSPCs by in vivo confocal and fluorescence microscopy (IVCM), quantitative RT-PCR (RT-qPCR), mass spectrometry, histology, and by measuring the IOP. To determine the mechanism by which HSPCs may rescue disease cells, we transplanted Ctns-/- mice with Ctns-/- DsRed HSPCs virally transduced to express functional CTNS-eGFP fusion protein.
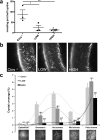
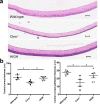
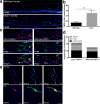
Results: We found that a single systemic transplantation of wild-type HSPCs prevented ocular pathology in the Ctns-/- mice. Engraftment-derived HSPCs were detected within the cornea, and also in the sclera, ciliary body, retina, choroid, and lens. Transplantation of HSPC led to substantial decreases in corneal cystine crystals, restoration of normal corneal thickness, and lowered IOP in mice with high levels of donor-derived cell engraftment. Finally, we found that HSPC-derived progeny differentiated into macrophages, which displayed tunneling nanotubes capable of transferring cystinosin-bearing lysosomes to diseased cells.
Conclusions: To our knowledge, this is the first demonstration that HSPCs can rescue hereditary corneal defects, and supports a new potential therapeutic strategy for treating ocular pathologies.
Figures


References
Publication types
MeSH terms
Grants and funding
- P30-EY022589/EY/NEI NIH HHS/United States
- DK076169-23789-5/DK/NIDDK NIH HHS/United States
- R01 NS081082/NS/NINDS NIH HHS/United States
- R01-DK099338/DK/NIDDK NIH HHS/United States
- P30 NS047101/NS/NINDS NIH HHS/United States
- P30 EY022589/EY/NEI NIH HHS/United States
- NS081082/NS/NINDS NIH HHS/United States
- R01-DK090058/DK/NIDDK NIH HHS/United States
- R01 DK090058/DK/NIDDK NIH HHS/United States
- R01 DK099338/DK/NIDDK NIH HHS/United States
- P30-NS047101/NS/NINDS NIH HHS/United States
- R21 NS090066/NS/NINDS NIH HHS/United States
- R21-NS090066/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

